Radiation Risk
By 1976, the Health Insurance Plan of New York study had begun to demonstrate the benefits of breast cancer screening. The Breast Cancer Detection Demonstration Project was under way when Bailar raised the concern that the radiation doses required for mammography in the 1960s might induce as many cancers as might be cured from early detection by mammography (1). This pessimistic evaluation generated a great deal of fear and resulted in a marked reduction in the use of mammography. A reassessment of the potential radiation risk to the breast was stimulated, as was research into techniques for reducing mammographic doses. The doses used for mammography are now much lower than those used by Bailar in his estimates as his estimates have been shown to be too high. Multiple reviews have concluded that the risk is very low and may even be nonexistent, certainly for women in the screening age groups of 40 and over. Nevertheless, despite years of evaluation and favorable analyses, some opponents of mammographic screening continue to raise questions about the safety of mammography (2,3).
Ionizing Radiation as A Carcinogen
Ionizing radiation is a known carcinogen. There is little question that exposure to high doses of radiation increases the risk of developing breast cancer. The likely mechanism is damage to chromosomal DNA from the free radicals that are produced when high-energy photons are absorbed in tissues. Chromosome breaks are known to occur from ionizing radiation.
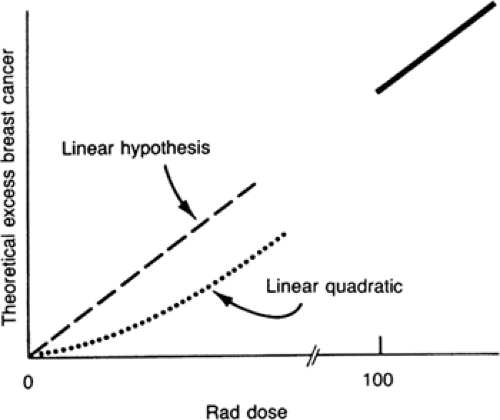
The effects of radiation are not uniform, however, and depend to a great extent on the organs being irradiated. Skin and bone, for example, are relatively insensitive to radiation, whereas blood cells are fairly sensitive. When assessing the risks of radiation, it is important to consider the organ(s) that have sustained the exposure. The effects on the breast have been shown to vary with dose and the age at which the woman is exposed (4). At high doses, the risk of radiation appears to be linear, with risk increasing in direct proportion to exposure. The relationship is less clear at lower doses. The available data can support a linear relationship or a linear quadratic relationship (5) (Fig. 5-1), although a 2003 review of women exposed at Hiroshima and Nagasaki suggested that the risk is linear even at low doses (4). The linear quadratic relationship begins with a lower risk at the lower-dose exposures than the straight linear relationship and at higher doses is very similar to the straight line model.
Disagreement has resulted from the uncertainties surrounding the possible effects (or lack of effect) associated with the low doses used for modern mammography. Bailar’s analysis was based on doses required to expose the high-detail, fine-grain industrial film that was used without intensifying screens in the 1960s and in the early years of mammography. Exposures at that time frequently exceeded several rads (cGy) per image. By the time radiation risk became an issue, doses had already been significantly reduced following the introduction of the xeroradiographic technique, which reduced doses to less than 2 rads (2 cGy) and eventually to less than 1 rad (1 cGy). Development of lower-dose film/screen combinations has lowered exposure requirements even further, so that modern film/screen systems require well under 0.2 rads (0.2 cGy) per exposure for an average-size breast. Digital mammographic units permit even lower exposures. Practitioners are cautioned, however, that efforts to lower the dose too much may result in a lack of photon flux and noisy images that can reduce the ability of the mammogram to reveal cancers.
There has never been any direct evidence that the doses required for modern mammography have any effect on the breast, particularly among women over the age of 40 in whom screening is beneficial. There is no scientific evidence that anyone ever has or ever will develop breast cancer as the result of a mammogram. All analyses of radiation carcinogenesis in the breast are based on extrapolation from populations exposed to doses that are at least two orders of magnitude higher than those required for x-ray mammography.
Evidence of Increased Risk of Breast Cancer from High-Dose Radiation Exposure
The evidence demonstrating the relationship of breast irradiation to excess cancer risk comes from studies of several groups of women who were exposed to moderate or large doses of radiation. The largest group included women among the more than 90,000 individuals who survived nuclear bombing but who were exposed to radiation at Hiroshima and Nagasaki (6,7). Another analysis comes from women who were treated with radiation for ankylosing spondylitis from the 1930s to the early 1950s (8). In the 1940s and 1950s approximately 600 women were treated with therapeutic doses of radiation (60 to 1,400 rads) for mastitis (9), and almost 40,000 women have been studied who underwent repeated chest fluoroscopies between 1930 and the early 1950s to monitor their pneumothorax treatment for tuberculosis (10,11,12). The preponderance of data from studying these women has shown a subsequent increased incidence of breast cancer among those exposed to high doses of radiation. More recent studies have shown a similar risk for women irradiated for Hodgkin’s disease (13,14,15). These studies leave little doubt that exposure to ionizing radiation increases the risk. However, debate continues over the magnitude of the risk and its applicability for those exposed to low doses. It has become increasingly clear that it is not simply the level of dose that determines the risk, but also the age of the individual at the time of breast exposure. At Hiroshima and Nagasaki, women whose breasts were exposed to the radiation when under the age of 20 were at a significantly increased risk; however, the risk dropped rapidly: women older than age 35 who were exposed had little or no increased risk (4). The same age relationships have been seen in women irradiated for Hodgkin’s disease (16). In one review 35% of women irradiated for Hodgkin’s disease while in their teens had developed breast cancer by age 40 (17). The risk that a Hodgkin’s survivor will develop breast cancer from the radiation decreases if the woman was irradiated in her twenties, and there is no demonstrably elevated risk if she was treated in her thirties or older. These findings clearly suggest that it is the undifferentiated, developing breast that is potentially susceptible to radiation carcinogenesis, while the “mature,” differentiated breast appears to have little if any radiation sensitivity with respect to carcinogenesis.
Genetic Abnormalities
Altered or damaged DNA appears to be the major mechanism involved in the development of breast cancers. There is some concern that women who already have, through the inheritance of damaged genes, abnormalities of DNA that predispose them to the development of malignancy may be at greater risk from carcinogens than the average individual. Some investigators have speculated that women who harbor the BRCA1 or BRCA2 gene may be at increased risk from these exposures. At this time, these concerns have not been validated and one study suggests there is no increased risk.
Ataxia-Telangiectasia Gene
One investigator raised a great deal of concern by suggesting that those who carry the ataxia-telangiectasia gene may be at increased risk. A 1991 report in the New England Journal of Medicine suggested that women who were heterozygotes (one abnormal copy) for the ataxia-telangiectasia gene were at increased risk for radiation-induced breast cancer. This was based on a case–control study of the relatives of homozygotes who had the syndrome. In this study the blood relatives (many presumed to be heterozygotes with one bad copy) of those with ataxia-telangiectasia who were exposed to diagnostic x-rays appeared to be at increased risk for developing breast cancer. The authors warned that because these heterozygotes were estimated to make up 1% of the general population, and because there is as yet no test to determine heterozygosity, all women should avoid breast radiation exposure (18). This concern was an extrapolation from the fact that homozygotes are known to be extremely sensitive to radiation. They postulated that the increased risk seen in homozygotes exposed to radiation would be reflected in their heterozygote relatives.
The conclusions of this study were based on a comparison of cases with unmatched controls and a very limited sample with serious flaws. Obligate heterozygotes (blood relatives of homozygotes have one bad copy of the gene) were questioned about their exposure to diagnostic x-ray studies, and the incidence of breast cancer among these women was then compared to spouses (no blood relationship), who were
less likely to harbor the gene. The authors found a greater number of breast cancers among the heterozygotes and raised the warning.
less likely to harbor the gene. The authors found a greater number of breast cancers among the heterozygotes and raised the warning.
Numerous letters to the editor disputed the authors’ con-clusions (19,20,21,22,23). Among the problems with the study, including the small sample size, was the lack of dosimetry. The actual radiation exposure to the breast could not be determined. Although the authors cautioned against mammography, exposure to mammography was not even determined in the study. The majority of the exposures were from angiographic studies that exposed the breasts to less than background radiation. A major bias was the fact that the study group was older than the controls (breast cancer incidence is higher in older women). Other risk factors were not accounted for, and the reasons for undergoing x-ray studies were not provided. The authors built their arguments on previous assumptions that they supported with their own previous work, which was similarly built on their own previous assumptions. The concerns raised by this paper are not supported scientifically and are likely incorrect. A study of the same individuals would likely have also shown that eating in the hospital cafeteria carried the same increased risk. Nevertheless, it is important to be alert to possible risks. It is likely that as we develop a greater understanding of the molecular biology of breast cancer, it will be possible to identify factors that predispose an individual and make her more sensitive to the many environmental carcinogens as well as radiation.
Significance of Radiation Risk
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree