MR imaging comprising high-resolution T2-weighted imaging and high b value diffusion-weighted imaging has proven effective in guiding treatment selection and preoperative planning for rectal cancer. In addition to staging, it enables the noninvasive assessment of key bioimaging markers such as extramural vascular invasion, tumor deposits, and the presence and location of mesorectal fascia and anal sphincter involvement. After neoadjuvant therapy, MR imaging offers noninvasive treatment response, complementing endoscopic and digital rectal evaluations. This assessment plays a crucial role in determining the feasibility of organ preservation or watch-and-wait strategy in patients who achieve complete clinical response.
Key points
- •
Dedicated pelvic MR imaging plays a crucial role in staging and restaging patients with rectal cancer, in surveillance of patients pursuing nonoperative management, and in assessing local disease recurrence following surgery.
- •
Optimization of MR imaging technique is essential for accurate interpretation. High-resolution T2-weighted imaging without fat saturation in an oblique axial plane perpendicular to the tumor base is critical for accurate initial staging.
- •
In the initial evaluation, MR imaging is used to provide clinical staging, assess imaging biomarkers, and distinguish between patients who are best suited for primary surgery and those who might benefit from neoadjuvant treatment.
- •
As part of restaging, MR imaging in conjunction with digital rectal examination and endoscopy is used to determine response assessment and identify patients who may benefit from nonoperative management.
- •
Evaluating locoregional lymph nodes in rectal cancer based solely on size criteria has limited sensitivity and specificity. To improve performance, and particularly to enhance specificity, the application of morphologic criteria is recommended at initial staging.
Introduction
In the late 90s, colorectal cancer was the fourth-leading cause of cancer death in both men and women younger than 50 years of age. However, it has recently become the leading cause of cancer death in men and the second leading cause in women under 50 years old. , Despite rectum being only one-tenth the length of the colon, adenocarcinoma of the rectum accounts for one-third of all colorectal cancers. In addition, rectal cancer carries a higher risk of positive resection margins and local recurrence compared to colon cancer, along with a distinct pattern of distant metastasis. Fortunately, high-resolution rectal MR imaging has proven to be effective in identifying patients at high risk for positive resection margins, enabling optimized treatment plans. Due to its superior soft tissue contrast, pelvic MR imaging using a tailored rectal cancer protocol is the preferred modality for local staging, restaging, and surveillance for those following a watch-and-wait strategy.
In this article, the authors aim to highlight the multidisciplinary approach, review current guidelines in staging, restaging, patterns of recurrence, posttreatment complications, pitfalls in image interpretations, and advances.
Imaging technique
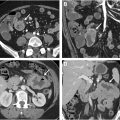
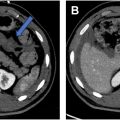
The bedrocks of a rectal MR imaging protocol are (1) multiplanar high-resolution T2-weighted imaging (T2WI), which provides an excellent depiction of the morphology and inter-relationship of the tumor, rectal wall, and adjacent mesorectal facia and (2) high b-value diffusion-weighted imaging (DWI), which helps to differentiate malignant from benign tissues and to detect residual viable tumors within the rectal wall or mesorectal facia after chemoradiation ( Fig. 1 A–H ). Intravenous contrast does not improve the overall diagnostic accuracy, however, postcontrast T1-weighted imaging (T1WI) is less affected by motion, and therefore can be helpful when high-resolution T2WI and DWI are of suboptimal quality. It may also help in differentiating T1 and T2 disease, and in the assessment in postneoadjuvant treatment response. When interpreting postcontrast images, it is important to correlate with T2WI series to minimize the risk of overestimating the disease extent ( Fig. 2 A–C ).


Patient Preparation and Scan Setup
MR imaging may be performed with either 3.0 or 1.5 T scanner. The patient should be instructed to fast for a few hours and to empty the bowel and bladder right before the examination. Administration of spasmolytic agent may help to control the rectal and bladder peristalses and reduce the related image ghosting and blurriness.
Multiplanar High-Resolution T2-Weighted Imaging
High-quality multiplanar T2WI with sufficient spatial and contrast resolution and signal-to-noise ratio (SNR) as well as minimal image blurring and artifacts is essential. Typically, this sequence is acquired in the sagittal, oblique axial (perpendicular to the tumor base at the rectal wall), and coronal or oblique coronal planes, respectively.
The high-resolution T2WI series should preferably be acquired with an in-plane resolution of less than 1 mm × 1 mm and a slice thickness of 2 to 4 mm with no slice gap ( Figs. 1–3 A–F ). To minimize ghosting from respiratory motion, the sagittal and oblique axial series should have the frequency encode direction set along the anterior/posterior direction. In addition, a judicious application of spatial saturation bands can be applied to further reduce the signals of any moving anatomies either outside (eg, at the anterior abdominal/pelvic wall of a sagittal series) or inside (eg, over the anterior abdominal/pelvic wall/urinary bladder for an oblique axial series) the imaging field of view (FOV). The acquisition time should be limited to 2 to 4 minutes per series to produce sufficient image SNR and minimize the potential for image degradation due to patient motion.

Small Field of View Diffusion-Weighted Imaging
DWI is most-commonly performed with a single shot echo planar imaging sequence and should be matched in FOV and slice thickness/slice coverage with those of the high-resolution oblique axial T2WI series. DWI with a high b-value of at least 800, preferably greater than 1500, along with apparent diffusion coefficient (ADC) map is recommended. Microenema may reduce the residual stool and bowel gas, limiting image distortion and artifacts.
Image interpretation—anatomy and pathology, initial staging, and restaging
Initial Staging
Primary tumor evaluation
Location
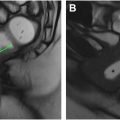
Understanding the location of the rectal tumor and various anatomic relationships is essential for selecting the optimal treatment approach. The first step is to measure the distance between the anal verge and the tumor, similar to how a rigid endoscope is used to determine the tumor location ( Fig. 4 A–C ). The anal verge is defined as the junction of the stratified squamous epithelium of the distal anal canal and the keratinized, hair-bearing squamous epithelium. It roughly corresponds to the inter-sphincteric groove, a palpable landmark for surgeons and gastroenterologist, and which appears on sagittal T2WI as a fat plane between the internal and external anal sphincters , ( Fig. 4 ). Traditionally, this distance has been used to classify tumors as low, mid, or high rectal tumor. However, more recently, alternative anatomic landmarks have been considered to account for variations in anorectal lengths between patients.

The next crucial step is to evaluate the relationships between the tumor and anal sphincter/anorectal junction and the anterior peritoneal reflection ( Fig. 4 ). The anorectal junction marks the proximal extent of the surgical anal canal and is defined by the upper margin of the puborectalis muscle ( Fig. 4 ). If the anus is involved, reporting should describe deepest radial plane and location as depending on extent, sphincter sparing may not be possible.
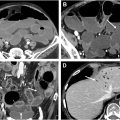
The anterior peritoneal reflection can usually be recognized on MR imaging. The portion of the rectum below the anterior reflection drains via the systemic pathway to the pelvic side wall lymph nodes and the portal pathway, making lower rectal tumors more susceptible to lateral lymph node involvement. Additionally, rectal tumors below the reflection are at an increased risk for positive resection margins ( Figs. 4 and 5 A–C ). Tumors involving the peritonealized portion of the rectum, on the other hand, are more at risk for peritoneal spread of disease.

T-category
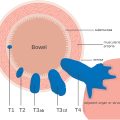
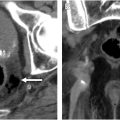
The T-category is determined by the depth of tumor invasion into or through the bowel wall: T1 invades the submucosa, T2 invades the muscularis propria, T3 invades the mesorectal fat, T4a invades the peritoneum without other adjacent organ involvement, and T4b invades adjacent organs or structures ( Fig. 6 A–D ). Properly oriented oblique axial T2WI without fat saturation, acquired perpendicular to the tumor base, is essential to prevent overstaging from T1/2 to T3 ( Fig. 3 ). For polypoid and ulcerated masses, it is important to note that the most advanced invasion occurs at the tumor base or ulceration, centered between the rolled edges ( Figs. 5 and 6 ). Polypoid tumors are generally lower in T-category compared to semi-annular or annular tumors. In early T1 cases, an intact submucosal stripe sign on postcontrast imaging, or intact submucosal layer on high-resolution T2WI may be detectable. However, MR imaging’s ability to differentiate between T1 and T2 is limited compared to endoscopic ultrasound and it is therefore recommended to seek and consider such clinical information at MR imaging interpretation. T3 substage is determined by depth of extramural penetration.

MR imaging is particularly effective in distinguishing good candidates for upfront surgery from those at high risk for positive resection margins. Ideal candidates for upfront surgery include upper rectal tumors, rectal tumors with extramural invasion less than 5 mm (T3a/b or lower category), and clear mesorectal fascia (MRF).
Mesorectal fascia
The MRF is the plane along which a total mesorectal excision (TME), the standard oncologic surgery for rectal cancer, most typically occurs. , The MRF circumferentially envelopes the extraperitoneal rectum and the surrounding mesorectal tissue below the anterior peritoneal reflection. At the level of the anterior peritoneal reflection, the anterior aspect of the mid rectum becomes peritonealized. As the upper rectum/sigmoid ascends in the pelvis, it becomes increasingly peritonealized laterally and eventually circumferential ( Fig. 5 ). If distance between tumor and MRF is 1 mm or less by MR imaging, the MRF is considered “involved”; if greater than 1 mm, it is “clear.” While not included in the TNM staging, MRF involvement has significant prognostic implications, as it is associated with positive resection margins and increased rates of local recurrence ( Fig. 7 A, B ). In radiology reports, when describing the tumor’s relationship with this anatomic structure, the term of “MRF” should be used rather the “curative resection margin (CRM).” Whereas the former refers to the anatomic structure seen at MR imaging, the latter refers to the pathologic specimen. They are not always congruent as the surgeon may modify the resection plane away from the MRF in order to achieve negative CRM.