Renovascular Hypertension: Endovascular Management
Thomas A. Sos
David W. Trost
Revascularization of renal artery stenosis (RAS) is indicated in the treatment of renovascular hypertension (RVH) and renal insufficiency (RI) due to ischemic nephropathy (IN); RVH and IN frequently coexist but may be present independently of each other. Hemodynamically significant RAS is one of the few potentially reversible causes of RI due to IN and hypertension. Recent multicenter prospective randomized trials, STAR (1), ASTRAL (2), and CORAL (3), have not shown any additional benefit for renal artery stenting in atheromatous disease over optimal medical therapy. The negative results of these trials have been criticized as being based on faulty methodology and statistics (4). The challenge for physicians is to identify patients with RAS who would benefit from renal revascularization, whether by interventional techniques or open surgery. In order to do so, RAS must first be clinically suspected and anatomically identified, and its hemodynamic significance and causal relationship to hypertension or RI must be documented. The risks and benefits of alternative medical and invasive therapies must be compared to each other and to the natural history of the disease.
Renovascular Hypertension
RVH accounts for about 5% of all hypertensive patients (5,6,7) and is usually due to atherosclerosis (75% of patients with RVH) or fibromuscular dysplasia (FMD) in the renal artery.
Angiographically documented RAS, greater than 70% diameter, and a history of sustained hypertension (140/95 mm Hg) in the setting of
1. Failed optimal medical therapy
2. Multiple antihypertensive agents required for blood pressure (BP) control (with an aim toward reducing, if not eliminating, the number of medications needed)
3. A strong clinical suspicion for RVH and at least one of the following:
a. A positive “Captopril Challenge Test” (plasma renin activity with angiotensinconverting enzyme inhibitor [ACEI])
b. A positive radionuclide renogram with ACEI (captopril or enalapril) challenge
c. Renal vein renin (RVR) secretion that lateralizes to one side with associated suppression of renin secretion from the uninvolved side (see “Renal Vein Renin Sampling”)
4. A mean arterial pressure gradient greater than 10% of the systemic BP across the stenotic segment of the renal artery (12). Areas of web-like stenosis that appear noncritical on angiography but have a significant systolic pressure gradient occur more often with FMD than with atherosclerosis. To measure gradients accurately in FMD, especially in the “string of beads” medial form, a pressure wire or a very flexible microcatheter is often necessary—a stiff angiographic catheter may “stent open” the soft fibrotic flaps responsible for the narrowing.
Note: Indications 1 through 3 may not be present in every case. Indication 4 must be present in every case.
Ischemic Nephropathy
IN usually results from bilateral atheromatous RAS; FMD virtually never produces IN. Many patients with IN have coexistent RVH. Revascularization may, especially in recent onset IN, reverse the process or prevent further decline of renal function.
Angiographically documented RAS greater than 70% in diameter and recent onset or deteriorating renal function while on optimal medical management and
Renovascular Hypertension, Ischemic Nephropathy, or Both, with Any of the Following Conditions
1. Renal transplant arterial stenosis: Most transplant renal artery stenoses are due to neointimal hyperplasia, accelerated atherosclerosis, clamp, or other iatrogenic injury usually at the perianastomotic area (9).
2. Renal artery venous, arterial, or synthetic bypass graft stenosis: These lesions occur most often at or adjacent to anastomoses and are due to perianastomotic fibrosis or clamp injury. Anastomoses must be examined in multiple projections to identify and quantify stenosis severity.
3. Recurrent flash pulmonary edema: These patients with RVH and/or azotemia usually have severe bilateral RAS rendering their kidneys unable to excrete sodium and water; they often do not have severe coronary artery disease (19).
Relative
1. Long-segment total occlusion (16)
2. Severely diseased aorta predisposing to increased risk of embolization of atheroma
Renal Artery Balloon Angioplasty
Preprocedure Preparation
1. Discontinue long-acting antihypertensive medications prior to procedure, if possible; manage BP with short-acting drugs as necessary (in consultation with managing physician).
2. In patients who already have RI or in those at increased risk such as diabetes, multiple myeloma, renal disease, and dehydration, hydrate overnight using 0.45% saline with sodium bicarbonate at a rate of 100 to 150 mL per hour for 4 to 12 hours prior to the procedure. If overnight hydration is not possible, at least 1 hour of hydration and N-acetylcysteine (600 mg twice daily on the day before and day of intervention) are also recommended (21,22,23). Consider using 50% or 30% dilute iodinated contrast or alternative contrast agents such as CO2. (For an in-depth discussion of periprocedural renal function management, see Chapter 65.)
4. Standard preangiography preparation
Procedure (Fig. 8.1)
1. Access to the (right preferred) common femoral artery: Almost all renal interventions can be performed from a femoral access. Place an arterial sheath (with a side arm for flushing). Patients who have significant iliofemoral atherosclerosis have a higher chance of distal cholesterol embolization. In this case, use a long (20 to 30 cm) arterial sheath, which reaches into the distal abdominal aorta, to minimize disruption of plaque during catheter exchanges and manipulations; in any case, a 40-cm long Flexor Ansel Sheath (Cook Medical Inc, Bloomington, IN) will usually be necessary later for intervention. Left brachial access can be used for patients with distal aortic occlusion or for those few patients who have a very unfavorable caudal renal artery angle for a femoral approach.
2. Diagnostic angiography: This should begin with a flush aortogram. Imaging needs to be performed in the proper oblique views to best visualize the lesion(s). Atherosclerotic patients have predominantly proximal and ostial disease, which are best imaged in 5- to 10-degree left anterior oblique (LAO) for the left renal artery and 20- to 30-degree LAO for the right renal artery (24). When no prior noninvasive imaging is available and both sides need to be evaluated, a compromise 20-degree LAO projection is preferred. Children or patients with suspected FMD should have selective magnification renal arteriography in at least two oblique views per side. When patients have significant bilateral stenoses, attempt angioplasty on the side with the larger kidney first
(usually also technically easier because disease tends to be less severe); if this goes well, and if the patient and operator can tolerate a prolonged procedure, attempt the other side.
(usually also technically easier because disease tends to be less severe); if this goes well, and if the patient and operator can tolerate a prolonged procedure, attempt the other side.
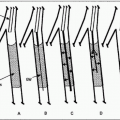
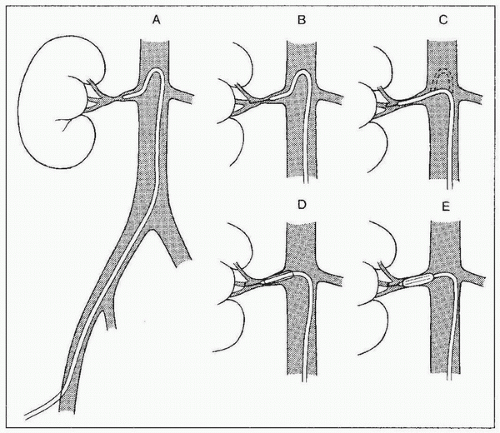 FIGURE 8.1 • Technique of renal angioplasty using shepherd’s crook (recurved) catheter. After selection of an appropriate renal artery (A), a flexible-tipped guidewire is advanced through the lesion under fluoroscopic control (B). The catheter is advanced across the stenosis by withdrawing the catheter at the puncture site (C). The guidewire is then exchanged for a heavy-duty, tight J-wire (D), and an appropriate balloon catheter is inserted to dilate the lesion (E). (Redrawn from Tegtmeyer CJ, Selby JB. Percutaneous transluminal angioplasty of the renal arteries. In: Castañeda-Zúñiga WP, Tadavarthy TM, eds. Interventional Radiology, Vol 2. 2nd ed. Baltimore, MD: Williams & Wilkins; 1992:370.) |
3. Crossing the lesion: In general, the stenosis should be crossed with a soft, atraumatic guidewire, such as a Bentson, and a recurved catheter such as a 4 Fr. Sos Omni Selective (AngioDynamics, Queensbury, NY) or a Simmons (Fig. 8.1). Aggressive/excessive catheter manipulation while finding the renal artery and crossing the stenosis can be the cause of cholesterol or macroparticle embolization. There are several published techniques to minimize this. The “no touch” technique described by Feldman et al. (25) minimizes the contact between the guiding catheter and the aortic wall, as does the “Sos flick” technique (26). Once the wire is across the lesion, the Sos Omni Selective catheter can be pulled down across the lesion. Hydrophilic guidewires should only be used as a last resort because they may cause inadvertent perforation or dissection and exchanged out as soon as practical. In most cases, 0.018 in. and 0.014 in. wire diameter and monorail/rapid exchange type designs are used. Frequently, after the 0.035 in. Bentson-type wire and Sos Omni Selective catheter have crossed, they are exchanged for a smaller diameter wire for intervention. Nitroglycerin (NTG) 100 to 200 µg intra-arterial (IA) into the renal artery can be given through the selective catheter before any guidewire insertion to
prevent spasm. If the wire advances with difficulty or the tip curves and is unable to be straightened, stop all wire manipulations. Assess whether the wire has passed subintimally or perforated. If occlusive dissection is present, prolonged (few minutes) under inflation with a balloon 1 mm lesser in diameter may restore the lumen. If occlusive dissection persists or perforation has occurred, a stent or covered stent may be necessary to complete the procedure. If the RAS is difficult to cross with the earlier techniques, consider using a different catheter/guidewire combination. If the renal artery has an extreme caudal angulation, consider an upper extremity approach. Once the catheter is safely across the stenosis, administer 5,000 U of heparin intravenously (IV) (70 U per kg).
prevent spasm. If the wire advances with difficulty or the tip curves and is unable to be straightened, stop all wire manipulations. Assess whether the wire has passed subintimally or perforated. If occlusive dissection is present, prolonged (few minutes) under inflation with a balloon 1 mm lesser in diameter may restore the lumen. If occlusive dissection persists or perforation has occurred, a stent or covered stent may be necessary to complete the procedure. If the RAS is difficult to cross with the earlier techniques, consider using a different catheter/guidewire combination. If the renal artery has an extreme caudal angulation, consider an upper extremity approach. Once the catheter is safely across the stenosis, administer 5,000 U of heparin intravenously (IV) (70 U per kg).
4. Pressure gradient across stenosis: A pressure gradient is considered to be significant if it is greater than 10% of the mean systemic arterial pressure (12). If there is not a significant gradient, then revascularization should not be continued. NTG 100 to 200 µg IA into the renal artery may provoke a gradient. Gradients should be measured with the lowest profile device to avoid inadvertently enhancing the severity of a stenosis. A 0.014 in. pressure wire is ideal; a 4 Fr. catheter clearly contributes more to an existing stenosis, but it is very useful in excluding unnecessary intervention in those patients who do not meet even this minimal threshold.
5. Balloon angioplasty (percutaneous transluminal renal angioplasty [PTRA]) (7,8,26,27,28,29) or stent placement (30,31,32,33,34,35,36,37,38): For ostial atherosclerotic stenosis, primary stent placement is indicated. If the lesion is non-ostial (greater than 1 cm from the origin), whether atherosclerotic or FMD, balloon angioplasty should be primarily performed. Primary stenting of FMD is contraindicated.
a. Choose a balloon diameter approximately 10% larger than the estimated “normal” diameter of the vessel based on the arteriogram. Do not be fooled by poststenotic dilation into choosing too large balloon size. If in doubt, use a smaller balloon, at least initially.
b. Some practitioners still use 5 Fr. balloon systems over a 0.035 in. guidewire; however, smaller sub-4 Fr. coronary-type balloon systems with 0.014 in. to 0.018 in. guidewires are being used with increasing frequency.
c. Guiding catheters or sheaths (5 to 8 Fr.) are used to provide support for crossing severe calcific stenosis and better seating within the ostium. They have a soft, blunt distal end, which minimizes arterial trauma but are almost as stiff as guiding catheters. The Flexor Ansel Sheath (Cook Medical Inc, Bloomington, IN) is supplied with two dilators: one tapered to a 0.035 in. guidewire and the other tapered to a 0.018 in. guidewire.
7. Dilation (see Fig. 8.1): Place a soft tip, stiff shaft guidewire into a distal branch for the intervention. Exchange the diagnostic catheter for the sheath/guide or directly for the balloon catheter. Prevent motion of the distal wire tip by firmly fixing it; otherwise, spasm may be provoked in the distal smaller vessels. Perforations can also occur if the guidewire is allowed to go too peripherally. Place the balloon markers across lesion.
8. Inflate the balloon slowly until the balloon is fully inflated or has reached its rated maximum pressure; for angioplasty, inflate for a minute, and for stenting, until stent is fully expanded. Discontinue balloon inflation if the patient experiences severe pain.
9. Deflate the balloon immediately and completely to avoid thrombus formation on balloon surface and possible vessel occlusion.
10. Remove the balloon catheter over the wire; prior to removal, it may be necessary to partially “refold” the deflated balloon by gently advancing the sheath/guide over its back end.
11. It is generally best to avoid recrossing the site of angioplasty after the vessel has been dilated. Do a completion angiogram using a technique that preserves wire position across the lesion. If a guide/sheath is present, the angiogram
can be obtained through the sheath. If there is no guide/sheath, the injection can be performed by using a 5 Fr. multi-side-hole catheter over the guidewire with the tip just into the renal artery and injecting through a side-arm adapter. A postangioplasty cleft is often seen and usually resolves in about 3 months (8).
can be obtained through the sheath. If there is no guide/sheath, the injection can be performed by using a 5 Fr. multi-side-hole catheter over the guidewire with the tip just into the renal artery and injecting through a side-arm adapter. A postangioplasty cleft is often seen and usually resolves in about 3 months (8).