Risk Stratification Using Inflammatory Markers and Other Risk Factors in Nuclear Imaging
Jack Rubinstein
George S. Abela
Risk Factors and Stratification in Chronic CAD
Research into risk factors for coronary artery disease (CAD) has continued to refine the stratification of patients with potential and diagnosed CAD.1 Innumerable reports have used myocardial perfusion imaging (MPI) to classify patient characteristics to better assess an individual’s risk for CAD. However, more recently, intense research has focused on a vast array of inflammatory markers that can potentially be used in conjunction with currently available clinical and radiologic findings. Despite the advances in the identification of inflammatory markers and their role in CAD, the current guidelines for prevention, detection, and evaluation of patients at risk for CAD do not recommend their routine use.2
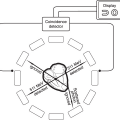
The ideal characteristics of a marker for coronary artery disease are as follows: First, a marker has to be identified as having a potential relation with endothelial damage or other inflammatory processes associated with CAD.5 Second, it should be inexpensive, easy to standardize, have clear cut-off points, and have a high sensitivity. Third, it should stand up to the scrutiny of population- based trials where its presence (or absence) has independent predictive value (Figure 7.1).6 Although some consider hs-CRP to be an exception, there is currently no marker available that fulfills all the above characteristics. However, algorithms have been developed that incorporate some of these markers to add to the predictive value of other forms of risk stratification.7
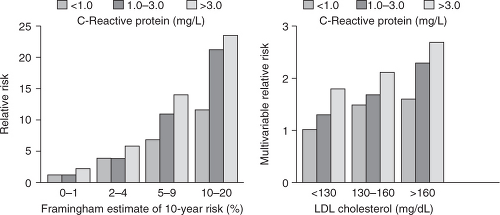
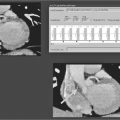
C-reactive protein (CRP) is a well-established acute phase reactant that has recently been identified as a major cardiovascular risk factor by using higher sensitivity assays (hs-CRP). It is expressed in smooth muscle cells within diseased arteries and has been found to play a role in atherogenesis and plaque vulnerability.10 It has prognostic value during acute ischemic events and in patients with established CAD. However, its most relevant use is in the setting of primary prevention. Several major studies have confirmed that it has an additive value to the Framingham risk score and to other markers such as low-density lipoprotein (LDL) cholesterol (Figure 7.2),11 though the degree to which it is predictive of CAD is still under discussion.12
Homocysteine
Increased concentration of homocysteine may result in vascular changes, including impairment of endothelial function, enhanced thrombogenesis, and smooth muscle cell proliferation.13 Also, elevated homocysteine levels have been correlated with CAD risk in some
case-control studies, but the data is not as clear in prospective studies.14 Although it has also been shown that folic acid supplementation reduces levels of homocysteine, its use has not been shown to decrease the risk of cardiovascular disease. In most cases the measurement of homocysteine levels is not indicated in the evaluation or risk stratification of patients with suspected or known CAD.
case-control studies, but the data is not as clear in prospective studies.14 Although it has also been shown that folic acid supplementation reduces levels of homocysteine, its use has not been shown to decrease the risk of cardiovascular disease. In most cases the measurement of homocysteine levels is not indicated in the evaluation or risk stratification of patients with suspected or known CAD.
Erythrocyte Sedimentation Rate15
Erythrocyte sedimentation rate (ESR) is a very sensitive marker of inflammation that has been extensively studied in various diseases. A meta-analysis of several population-based studies using ESR concluded that there was mildly relevant—but statistically significant—use for it as a marker for CAD. Based on these studies, the use of ESR is not advocated in further classifying patients for potential CAD.
Others
Fibrinogen16
Fibrinogen is very relevant in the clot formation process. It is also an acute phase reactant that has been studied as a possible marker for CAD. In several studies it was demonstrated to have predictive value. However, it has not been accepted into routine clinical practice because of the difficultly in standardization.
Interleukin-617
Interleukin-6 (IL-6) is a powerful inducer of the hepatic acute phase response and is the primary determinant for C-reactive protein production. IL-6 decreases lipoprotein lipase (LPL) activity in plasma, which increases macrophage uptake of lipids. Macrophage foam cells and smooth muscle cells (SMC) express IL-6, suggesting a role for this cytokine along with interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) in the progression of atherosclerosis.18 In prospective studies it has been shown to be mildly predictive of future coronary events in apparently healthy populations,19,20 though its prognostic value over other markers has not been clearly established because of its high variability.
Lipoprotein Associated Phospholipase A2
Lipoprotein associated phospholipase A2 (Lp-PLA2) is secreted by macrophages, T-cells, and mast cells and is transported through the body mostly via LDL. Over-expression of Lp-PLA2 has been found in different populations to both increase and decrease risk for CAD, and animal studies have suggested that it is mostly a pro-atherogenic protein. Research is still ongoing and most agree that additional data are needed to elucidate its role.
Plasminogen activator inhibitor-1 (PAI-1) has been shown to exert a pro-atherothrombotic effect on endothelial cells. There are epidemiological studies that PAI-1 excess may contribute to the development of ischemic cardiovascular disease. In vivo administration of antibodies against PAI-1 has been proven effective in preventing development of thrombus in transgenic mice expressing human PAI-1 develop coronary arterial thrombosis.23 To date, PAI-1 has not been used as a marker for stable coronary artery disease, but preliminary data indicates that it may be useful at the time surrounding an acute coronary event as plasma PAI-1 activity has been noted to be increased in MI survivors that later develop recurrent MI.
Imaging Findings
Beyond the finding of ischemia during MPI there are other less commonly used but potentially useful indicators of CAD, such as ejection fraction and ischemic dilatation. These findings are particularly applicable in high and medium risk patients that have mild to moderate ischemia.
Left Ventricular Ejection Fraction
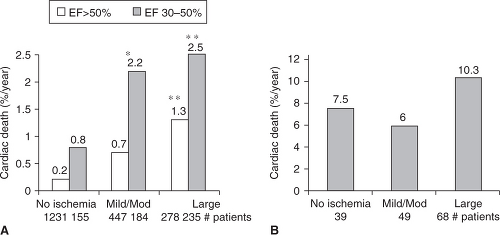
It is well established that ejection fraction (EF) is inversely related with cardiac mortality. With the assistance of currently available software, it is now commonplace to evaluate ejection fraction along with perfusion during a standard MPI. This “added” information should be considered in the overall cardiac evaluation, as post-stress EF seems to be closely related to the likelihood of cardiac death (Figure 7.3).24
Number of Reversible Defects
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree