The sacroiliac joint (SIJ) is the largest diarthrodial joint in the human body and accounts for approximately 20% of all low back pain, which is commonly seen in patients with lumbosacral fusions. Despite this, SIJ dysfunction often poses a challenging diagnosis depending on clinical evaluation, imaging, and image-guided joint injection. SIJ fusion is an effective and safe method of treatment, with minimally invasive approaches fitting well within the armamentarium of interventional radiologists treating chronic pain and other musculoskeletal conditions. Contemporary technical approaches and clinical considerations are discussed.
Introduction
Historically neglected as a clinically relevant structure relative to other components of the axial skeleton, the sacroiliac joint (SIJ) has gained renewed interest in recent years as an important pain generator and target for minimally invasive treatment. In stark contrast to the initial attempted SIJ fusion in 1908 utilizing bone graft and an open approach, modern treatment predominantly consists of image-guided placement of specialized fusion devices through minimally invasive and even percutaneous methods, making such techniques a logical addition to the armamentarium of musculoskeletal interventional radiologists. More importantly, the proper diagnosis of SIJ dysfunction often remains elusive; the combined aspects of awareness of the entity, imaging evaluation, clinical examination, and firm diagnosis with image-guided joint injection provide an important opportunity for clinicians of our specialty to provide comprehensive care to patients suffering from this often-debilitating condition.
As an anatomic structure, the SIJ is quite complex both morphologically and mechanically. It serves as a crucial transition zone between the axial and appendicular skeleton, transferring the compressive load from the lumbosacral spine to the iliac bones and thereby to the hips. Despite potential movement in several planes, consisting of flexion-extension, nutation-counternutation, axial rotation, lateral bending, and translation, physiologic movement of the joint is relatively minor in any direction, on the order of 1.4-3.1 mm. Pathologic states driven by increased stress or laxity of the joint can contribute to pain through its innervation, primarily via the medial branch dorsal rami of S1-S3, which may be experienced as pain in the low back, gluteal region, hip, or even the posterior lower extremities (a type of pseudosciatica likely driven by irritation of the lumbosacral trunk that lies just ventral to the joint). Pain is often bilateral. Due to its varied manifestations, the diagnosis of SIJ dysfunction can be difficult and is often attributed improperly to other entities such as facetogenic back pain, hip osteoarthritis, piriformis syndrome, and lumbar radiculopathy, among others. Overall, the SIJ is estimated to account for 15%-30% of low back pain.
Complicating the treatment of SIJ dysfunction are myriad factors including frequent anatomical variation, with Vereecke et al. recently reporting nonconventional SIJ anatomy in approximately 80% of patients, meaning that variation from so-called conventional anatomy is the norm rather than the exception. Among these variants are transitional lumbosacral anatomy (potentially confounding accurate segmental delineation and possibly accounting for increased stress on the SIJ), accessory articulations, synostoses, and variant iliac anatomy, all of which may complicate or preclude certain technical approaches to the joint for both injections and intra-articular fusions. Such factors make the imaging evaluation crucial in treatment planning but there are no sensitive or specific imaging features diagnostic for SIJ dysfunction, meaning that the diagnosis is almost entirely clinical, beginning with consideration of the entity based on history, targeted physical examination, and subsequent image-guided SI injection to confirm the pain generator.
The treatment options for properly diagnosed SIJ dysfunction consist of a growing continuum, ranging from physical therapy (PT) for strengthening supporting muscular and ligamentous structures, to therapeutic joint injections for temporary relief, to thermal ablation for temporary denervation of the joint (mostly via radiofrequency (RFA), although cryoablation has recently been suggested as a valid modality), and ultimately to fixation and fusion for a hopefully durable solution.
Recently in the US, most Medicare Administrative Contractors (MACs) no longer consider RFA of the SIJ to be medically necessary, making this treatment option less and less economically viable for American practitioners despite support by multiple RCTs and metaanalyses. While the evidence is positive for RFA, the durability of relief has consistently shown to be temporary on the order of months. Endoscopic neurotomy has been suggested as a more durable method, but this treatment is not yet supported by clinical trials or economic framework in the American healthcare system. The current status of cryoneurolysis under a temporary T-code in the CPT lexicon and current lack of clinical trials similarly make the future utility of cryoablation for SIJ dysfunction uncertain at this time.
These myriad factors and the continual development of specialized sacroiliac devices have led to increasing use of minimally invasive fusion as a durable solution for SIJ dysfunction. Compared to historical methods consisting of open approaches with associated prolonged recovery and undue morbidity, modern SIJ fusion devices tend to be minimally invasive or percutaneous. Numerous approaches to fuse the joint have been described, with most falling into categorization as lateral iliosacral, posterolateral iliosacral, or posterior/dorsal intraarticular. The remainder of this review will focus on these 3 general approaches to SIJ fusion and how they can fit into the interventionist’s armamentarium, with the obvious caveat that manufacturers’ specific products often have specific technical elements germane to their devices.
Clinical evaluation of the patient
Perhaps most important in the initial evaluation of the SIJ as a potential pain generator is the recognition of important predisposing clinical factors in the patient’s history, with the most frequent being prior lower lumbar or lumbosacral fusion, history of childbirth, or history of high-velocity trauma. An L4-S1 fusion contributes to a 168% increase in motion on the SIJ, compared to 52% added motion from a monosegmental L5-S1 fusion, but even lower lumbar fusion constructs not incorporating S1 increase the development of SIJ dysfunction, with an L4-L5 instrumented fusion increasing stress on the SIJ by a lesser but significant degree. As such, SIJ dysfunction should remain in the differential for any patient with a prior fusion experiencing LBP, buttock pain, hip pain, or leg pain.
While postpartum pelvic instability is classically attributed to multiparity, owing to the cumulative effect of physiologic release of relaxin on ligamentous laxity in multiple pregnancies, patients can certainly develop postpartum SIJ dysfunction after a single pregnancy, so any history of gravidity should prompt consideration. A history of high velocity trauma can also be a predisposing factor even without discrete injury to the bony pelvic ring, presumably resulting from radiologically occult damage to ligamentous structures.
Degenerative osteoarthritis of the sacroiliac joint (often referred to as degenerative sacroiliitis when symptomatic) on its own can be an etiology of SIJ dysfunction and is seen increasingly with advancing age, but symptomatic SIJ degeneration generally is seen in the elderly population much more frequently in patients with prior lumbar fusion than without, for the biomechanical reasons previously mentioned. As such, the presence of imaging features of SIJ degeneration (including subchondral sclerosis, cysts, or erosions, joint air, and osteophytes) are important findings as in the assessment of any arthritic joint, but not necessarily specific findings of symptomatic SIJ dysfunction.
Finally, and in a somewhat different category because of the fundamental difference in management, inflammatory spondyloarthropathies are important contributors to SIJ pain. Of this broad disease class, the most common is ankylosing spondylitis (AS), followed by other seronegative spondyloarthropathies. Patients tend to have multijoint involvement and present at a younger age (more common in people ages 17-35) than patients with age-related osteoarthritis, often with no identifiable imaging markers of arthropathy. Despite this, physical examination may elicit pain from the SIJ, and arthropathy can be further corroborated by elevated erythrocyte sedimentation rate (ESR) and by imaging findings of sacroiliitis including subchondral edema and enhancement on MRI, as well as periarticular radiotracer uptake on Tc99-MDP bone scan (with the periarticular localization well demonstrated on SPECT/CT). Symmetric bilateral findings further suggest inflammatory rather than infectious arthropathy. Other associated findings of AS may be apparent on lumbar imaging, including Romanus lesions, vertebral squaring, dural ectasia, and syndesmophyte formation. Such findings should prompt the consideration of seronegative spondyloarthropathy in a patient without other risk factors for SIJ dysfunction. Primary treatment with SIJ fusion is generally ill-advised in these patients given the systemic inflammatory nature of the underlying condition, which merits a rheumatologic evaluation and consideration of medical therapies including NSAIDs, anti-TNF alpha biologic agents, IL-17A inhibitors, or disease-modifying anti-rheumatic medicines (DMARDs). In general, the symptoms of inflammatory spondyloarthropathies become less prominent with age (commonly referred to as “burning out”), with AS often yielding sacroiliac autofusion and confluent syndesmophytes (the “bamboo spine” radiographic sign) on account of the osteoproductive nature of the condition. Patients who continue to have SIJ pain despite optimization of medical therapy can be treated with fusion, with the caveat that these patients may not experience the same degree of relief compared to those with mechanical SIJ dysfunction, on account of the inflammatory nature of the condition.
Any patient with LBP, buttock, hip, or leg pain and risk factors for SIJ dysfunction should undergo physical examination to elicit pain from the SIJ joint. Fortin’s finger test consists of the patient localizing pain to the PSIS, which will elicit pain on palpation; however, this test is nonspecific and must be further corroborated by further examination. The most frequently used testing cluster consists of 5 or 6 maneuvers including pelvic distraction (gapping), pelvic compression (approximation), thigh thrust/posterior shear, sacral thrust, Gaenslen’s test/pelvic torsion, and Faber’s test. Detail on these examinations is abbreviated for this article but freely available online. Provocation of the patient’s characteristic pain with at least 3 of these maneuvers (Fortin’s finger test being excluded) is considered a necessary component of the diagnosis.
Having established sufficient suspicion for SIJ dysfunction, the diagnosis is confirmed with image-guided injection of the SIJ yielding majority relief of the patient’s pain. Various cutoff values have been proposed, with 75% pain relief commonly being considered adequate to confidently identify the SIJ as the pain generator. Possible modalities of image-guidance include fluoroscopy, CT, and ultrasound, with fluoroscopy most commonly used for its availability and versatility.
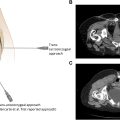
In the fluoroscopic method, a pelvic outlet view (AP with 20-30 degrees cranial angulation, projecting the sacrum along its long axis) with slight contralateral oblique angulation relative to the joint of interest provides a clear window to the inferior aspect of the joint lateral to the S2 foramen. The joint is centered under the fluoroscope to avoid parallax. A more posteroinferior trajectory to the inferior aspect of the joint at S2 can be facilitated using a more caudal angulation approaching a pelvic inlet view ( Fig. 1 ). A 22-gauge Quincke spinal needle (typically 3.5-inch, though 5-inch needle may be necessary for larger patients) is advanced into the joint from a medial start position, just lateral to midline. A characteristic tactile sensation may be appreciated on entering the joint, but ventral position of the needle can be assessed either with a standard lateral projection or a pelvic inlet view if needed. Intraarticular position of the needle is confirmed with injection of 0.5-1 cc of iodinated contrast. The inferior aspect of the joint is most easily accessed owing to the lack of ligamentous extraarticular recess at this level, as well as the more medial and posterior position relative to the more superior S1 segment, where access to the joint may be complicated by the superimposed PSIS. An alternate approach to the S1 segment can be performed from an identical view starting medially at the level of the S1 foramen and driving the needle anterolaterally deep to the posterior superior iliac spine (PSIS), through the extraarticular recess, and into the articular portion of the joint at this level. This technique may be more difficult to master initially given that the PSIS usually blocks a “down the barrel” trajectory, but anecdotally has been described as yielding superior SIJ arthrograms. CT guidance is considerably more straightforward ( Fig. 2 ) but more time-consuming, while ultrasound guidance is less commonly used if either CT or fluoroscopy are available.


Regardless of modality, confirmation of intraarticular position should be followed by injection of either local anesthetic alone (diagnostic injection) or anesthetic plus steroid (therapeutic injection). Notably, different insurance carriers may mandate certain volumes or mixtures of injectates and may stipulate that a diagnostic injection with anesthetic alone must be performed prior to any therapeutic injection, and that a second “confirmatory” diagnostic injection must be performed prior to proceeding to any therapeutic interventions, despite the dearth of any evidence to support this stepwise progression. Individual clinicians may have their own preferences based on such logistical barriers and other factors, but 1 possible injectate consists of 40 mg/1 cc of triamcinalone, 1 cc lidocaine without epinephrine, and 3 cc of 0.25% bupivacaine split between the articular and extraarticular compartments.
Degree of pain relief is assessed either immediately or shortly after the procedure depending on the patient’s degree of sedation and determines suitability for further interventions.
If not already obtained, noncontrast CT of the pelvis is important to assess the entire SIJ anatomy for treatment planning and is increasingly considered a requisite for treatment by insurers, ostensibly to exclude fracture, tumor, or other anatomic explanation for the patient’s pain.
Indications for the procedure
The indications for SIJ fusion are the same as the diagnostic criteria for SIJ dysfunction discussed above, with failure to respond durably (usually for at least 6 months) to more conservative treatment including analgesics, PT, and injections. Again, insurance criteria are variable across payors, and specific policies may apply more stringent criteria.
Contraindications are mostly general, including inability to temporarily discontinue anticoagulants or antiplatelet agents, or the presence of tumor or fracture (which would supersede SIJ dysfunction as a target for treatment). Additionally, patient factors that can impede bone healing must be considered as at least relative contraindications, including osteoporosis (which may benefit from initial treatment with anabolic bone agents prior to fusion), smoking, active regular NSAID use, and chronic systemic corticosteroid exposure. These factors should be optimized. An important specific relative contraindication is the desire for future childbearing, which physiologically depends on sacroiliac and pubic symphyseal joint laxity to accommodate the gravid uterus and childbirth. A dearth of data exists on the subject, but these basic biomechanical considerations indicate the importance of discussion of the topic in treatment planning with premenopausal patients.
Equipment needed
The primary 3 approaches to minimally invasive SIJ fusion are posteroanterior (commonly called posterior or dorsal), posterolateral transiliac, and lateral transiliac. The latter 2 types are considered transfixing approaches as they cross and fixate the joint and are typically performed using specialized screws or similar osteoconductive implants. Posterior approaches are generally considered interpositional techniques, employing distraction arthrodesis by placing an intraarticular device to bridge the joint, most frequently consisting of a bone allograft, although some products use a posterior approach to place an intraarticular screw or other specialized fusion device. In general, the device manufacturer’s kit should contain all the necessary equipment for the procedure, apart from basic surgical equipment including scalpels, electrocautery, local anesthetic, and possibly rigid bone access needle, if desired.
The lateral and posterolateral approaches provide biomechanically similar results through transfixation and eventual fusion of the joint, differing in procedural steps and fluoroscopic approach. The implants for these approaches tend to be similar or identical to each other, increasingly consisting of 3D-printed porous and osteoconductive screws with self-auguring mechanisms, rather than more primitive machined screws. Because these are transfixing approaches, they are coded differently (CPT 27279) and have different site of service stipulations (hospital outpatient department or ambulatory surgical center) than posterior approaches, which were recently granted a separate CPT code (27278) and may be usable in an office setting under moderate sedation, in addition to HOPD and ASC. Therefore, the choice of approach depends on multiple factors including patient anatomy (with some SIJs not amenable to a posterior approach), comorbidities (a posterior approach under moderate sedation perhaps being preferable to a transfixing approach under general anesthesia or deep sedation for a more elderly and frailer patient), local resources including OR time and anesthesia staff, and physician comfort with a given approach and product. These factors should all be weighed and discussed with the patient.
Procedural steps
Lateral approach
The patient is placed prone and general anesthesia or deep sedation/monitored anesthesia care is induced. Hands are placed toward the head, and generous padding is provided to cushion the face, pelvis, and extremities. In the case of fluoroscopic guidance which is most commonly used, the C-arm is positioned opposite the operative side and should be able to easily move between pelvic outlet, pelvic inlet, and lateral projections without catching on the bed or other obstacles, or interfering with the placement of the guidepin and other implements necessary for the procedure. The patient is widely prepped along the entire lateral aspect of the hip from above the iliac crest to the upper thigh (with generous prepping posteriorly along the buttock) and draped in the standard sterile fashion with only the lateral operative field exposed.
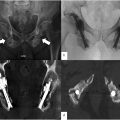
An initial lateral projection is obtained and optimized ( Fig. 3 ) by utilizing slight obliquity or “wig-wag” as necessary to superimpose the bilateral sacral ala and the sciatic notches. From here, the long axis of the sacrum is marked on the skin by overlying a guidepin from the superior endplate of S1 to the inferior aspect of S3 to indicate the cranial, caudal, ventral, and dorsal aspects, which differ from those of the lumbar spine due to the sacrum’s dorsal tilt. The ventral and posterior aspects of the sacral body can similarly be marked, and the alar line can also be marked to indicate a superior boundary for the superior screw. The S1 superior endplate and vestigial S1-S2 disc can be marked to define the S1 and S2 bony corridors. Following local anesthetic, a 1.5-3 cm longitudinal skin incision is made centered at the level of the S1 foramen (indicated by the vestigial S1-S2 disc on the lateral projection) using a 10-blade scalpel. Blunt dissection and manual tamponade or electrocautery are used to obtain superficial hemostasis prior to insertion of the first guidepin.