Setup and Field Arrangement for Conventional Technique
Target volume 5 cm or less deep (superficial lobe and deep lobe in thin patients)
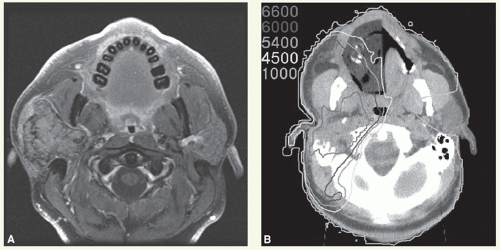
(see Case Studies 13-1 and 13-2).An intraoral stent containing cerrobend is used to shield the posterior oral tongue (see
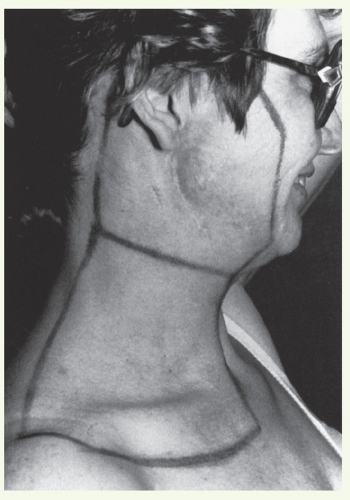
Chapter 3).Marking of the surgical scar and lateral canthus of the ipsilateral orbit facilitates portal design.
The patient is immobilized in an open-neck position with a thermoplastic mask. Flattening of the ipsilateral ear against the mastoid process minimizes dose heterogeneity resulting from electron perturbation. For the same reason, the external auditory canal is filled with Domeboro fluid prior to each electron treatment.
Bolus is used to cover the superior aspect of the portal when it extends above the zygomatic arch to minimize the dose to the temporal lobe of the brain.
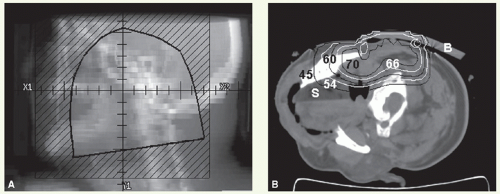
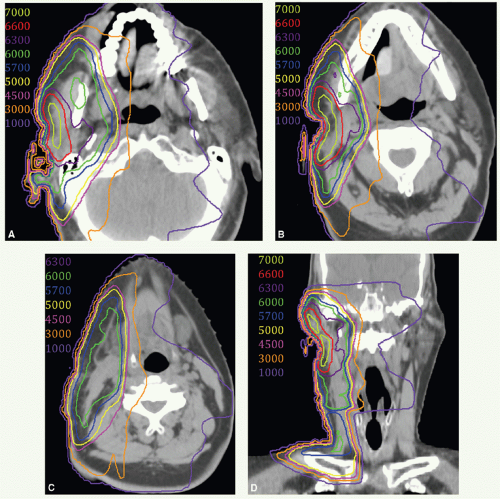
A lateral appositional field is used to cover the parotid bed and upper neck nodes. Radiation is delivered with a combination of electrons (12 to 20 MeV depending on the depth) and photons (6 MV) usually in the ratio of 4:1.
Portal borders are as follows:
Superior: zygomatic arch or higher as indicated by tumor extent or surgical scar.
Anterior: anterior edge of the masseter muscle.
Inferior: thyroid notch.
Posterior: just behind the mastoid process.
A 1.5- to 2-cm bevelled bolus or a computer-generated custom bolus is placed at the superior part of the portal (above the line connecting the orbital floor and the mastoid process) to reduce the dose to the temporal lobe. A lateral appositional electron field is used to treat the mid and lower neck nodes when indicated (for borders of neck field, see “General Principles”). Field reduction takes place after 50 to 54 Gy in 25 to 27 fractions to deliver the boost dose when indicated. If the anterior edge of the portal is close to the eye, skin collimation is applied and the beam maybe angled 5 to 10 degrees posteriorly to minimize the dose to the orbital content.
For more deep-seated tumors or when the facial canal is part of the target volume, a wedge-pair technique
(see Case Studies 13-3 and 13-4) or intensity-modulated radiation therapy (IMRT)
(see Case Studies 13-5, 13-6 and 13-7) with photon beams is often preferable.
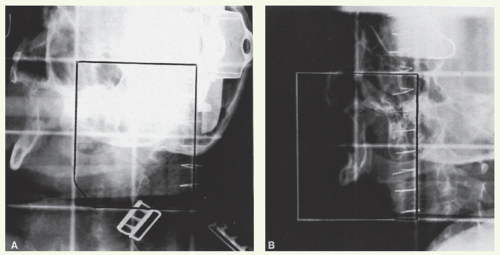
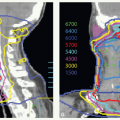
With these techniques, the patient is immobilized in a supine position with the head hyperextended with thermoplastic mask. The axial plane of the fields is chosen so that the posterolateral portal does not exit through the contralateral eye. A relatively simple wedge-pair technique uses anterolateral and posterolateral oblique photon fields (the anterolateral oblique field is on the spinal cord and the posterolateral oblique field is off the cord). The simulation focuses on marking of the surgical scar and both inferior orbital rims and selection of provisional isocenter. The provisional isocenter is generally placed at the center of the square defined by the zygomatic arch, anterior edge of the masseter, thyroid notch, and mastoid, and halfway between the skin and the oropharyngeal wall.
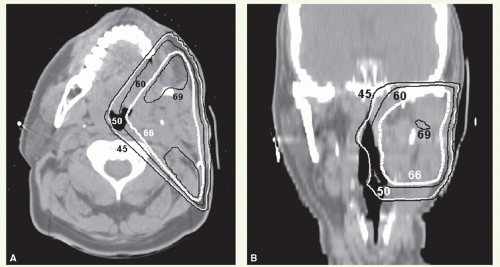
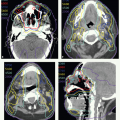
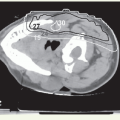
Thin-slice computed tomography (CT) scan is obtained in the treatment position for outlining the target volume and planning the portal sizes, hinge angle, and thickness of wedges using treatment planning system. No off-spinal cord reduction is required with a wedged-pair technique because the posterolateral field is off the cord from the beginning. Field reduction for boost dose, when indicated, occurs after 50 to 54 Gy.
Virtual Gross Target Volume
There is no actual gross target volume (GTV) after complete surgical tumor resection. However, it can be useful to formulate a virtual GTV (vGTV) to facilitate target volume definition. The vGTV is a best approximation of the
tissues having high likelihood of harboring microscopic tumor reconstructed based on findings of preoperative clinical examination, imaging studies, and surgical-pathologic assessment.