Abstract
Pseudoaneurysms of the splenic artery usually arise as a complication of pancreatitis. Due to the risk of rupture, treatment of the pseudoaneurysms of splenic artery is considered as a priority in the management of pancreatitis. While endovascular embolization is an established minimally invasive and effective technique for the treatment of splenic artery pseudoaneurysms, however, in some cases endovascular embolization may not be feasible, owing to the difficulties in accessing the distal small pseudoaneurysms or due to financial constraints. In such a scenario, percutaneous image guided direct puncture and embolization of the pseudoaneurysms is a valuable option. While most of the previous publications have reported on the use of n‑butyl cyanoacrylate, coils and thrombin for percutaneous embolization of splenic artery pseudoaneurysms, however, these agents may not be easily accessible to many health facilities in emergency situations and their cost may limit their use. In this report, we describe a novel technique of percutaneous embolization of splenic artery pseudoaneurysms with S equential A spiration and injection of a M ixture of gelatin-based hemostatic agent and autologous blood clot followed by suture coiling for E ffective E mbolization of splenic artery pseudoaneurysm by interventional R adiologist (SAMEER technique). We demonstrate the safety and efficacy of this technique in a series of 2 cases.
Introduction
Splenic artery is the most commonly affected visceral artery and is ranked as the third most common intra-abdominal artery to be affected by aneurysmal pathology [ ]. Most of the cases of splenic artery pseudoaneurysms are associated with pancreatitis [ ]. In the clinical scenario of acute pancreatitis, enzymatic degeneration of the wall of the splenic artery by the inflammatory process or associated complications, such as walled-off pancreatic necrosis, predispose to the development of pseudoaneurysms of the splenic artery, which has a close anatomical proximity to the pancreas [ ]. Since pseudoaneurysms lack a well-defined wall, they are predisposed to rupture and may lead to hypotension and shock [ ]. In rare cases, the pseudoaneurysms may rupture into the pancreatic duct to cause upper gastrointestinal hemorrhage, a condition referred to as Hemosuccus pancreaticus [ ]. Conventionally, the splenic artery pseudoaneurysms have been managed with open surgical or laproscopic approaches [ ]. However, an acute inflammatory process associated with acute pancreatitis, could pose technical difficulties in reaching upto the site of the pseudoaneurysm and may lead to failure of treatment or additional complications [ ]. In sub-acute to chronic stages, fibrotic changes in the pancreatic bed may also preclude successful surgical management of pseudoaneurysms [ ]. Recently, endovascular approach has been advocated as a safe method of treatment of splenic artery pseudoaneurysms . Endovascular coil embolization or embolization with liquid embolic agents have been shown to be safe and effective in obliteration of the splenic artery pseudoaneurysms [ ]. However, in many cases, endovascular approach to splenic artery may not be feasible, largely due to difficulties in access to the pseudoaneurysm as splenic artery may exhibit tortuous course and may pose difficulties in reaching the pseudoaneurysms of the distal branches of the splenic artery. In such cases, percutaneous direct embolization of the pseudoaneurysm has been shown to be a safe alternative treatment [ ]. While previous cases of percutaneous direct embolization of splenic artery pseudoaneurysms have been carried out with various embolic agents such as thrombin and n‑butyl cyanoacrylate glue, we describe a novel and cost effective technique of direct percutaneous embolization of splenic artery pseudoaneurysms in the clinical context of acute pancreatitis using gelatin-based hemostatic agent and autologous blood clot mixture, followed by coiling the aneurysm sac with nylon sutures to effectively embolize the pseudoaneurysm sac. This technique is termed as S equential A spiration and injection of a M ixture of gelatin-based hemostatic agent and autologous blood clot followed by suture coiling for E ffective E mbolization of splenic artery pseudoaneurysm by interventional R adiologist technique (Acronymized as: SAMEER technique).
Case report
We hereby describe the SAMEER technique with the help of a series of 2 cases of acute pancreatitis complicated by splenic artery pseudoaneurysm arising from the distal (splenic hilar) splenic artery branch. In both the cases, the tortuous course of the splenic artery and location of the pseudoaneurysm were considered as a potential source of difficulty and failure of endovascular embolization of the pseudoaneurysms. Also, considering the resource poor condition of the respective families, in both the cases, a novel technique was needed to tide over the emergent health condition of ruptured splenic artery pseudoaneurysms.
Description of the SAMEER technique
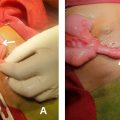
The basic tenet of the SAMEER technique is to puncture the sac of the pseudoaneurysm using an 18 G puncture needle under imaging guidance. The modality used to puncture the sac of the pseudoaneurysm depends on how well the pseudoaneurysm is visualized on that particular modality. In this regard, ultrasound or CT may be used to puncture the pseudoaneurysm. Care must be taken to puncture the pseudoaneurysm in a single attempt as multiple attempts may lead to intra-procedural rupture of the pseudoaneurysm. The materials required for the technique are summarized in Table 1 .
| Serial No. | Material |
|---|---|
| 1. | 18 G puncture needle |
| 2. | Gelatin-based hemostatic agent |
| 3. | 10 mL Leur lock syringes |
| 4. | 3-way stop cock |
| 5. | Normal saline (100 mL) |
| 6. | 2-0 Nylon sutures |
| 7. | Iodinated contrast agent |
The steps of the SAMEER technique are as follows.
- 1.
Identify the dome of the pseudoaneurysm using the imaging modality chosen by the operator.
- 2.
Using imaging guidance, puncture the pseudoaneurysm with 18 G puncture needle
- 3.
Once the position of the needle is confirmed within the sac of the pseudoaneurysm, free back flow of arterial blood is obtained.
- 4.
Aspirate approximately 5 mL of arterial blood into a 10 mL leur lock syringe and allow the blood in the syringe to partially clot. While the blood is allowed to clot in the syringe, the hub of the puncture needle is occluded to avoid blood loss.
- 5.
While the blood is allowed to form a coagulum, a slurry of hemostatic gelatin-based agent with normal saline and iodinated contrast mixture is prepared, to make a volume of about 5 mL. The slurry is made in a standard manner by cutting the gelatin-based hemostatic agent into small pieces and placing the pieces in a 10 mL leur lock syringe and vigorously mixing this with 5 mL of normal saline and iodinated contrast solution in another syringe, while connecting the 2 syringes to a 3-way stop cock and agitating the mixture for about 50-60 passes to make a semi-solid slurry.
- 6.
In approximately 5-10 minutes, once the withdrawn blood in the syringe is in a semi-solid state, using a 3-way stopcock, the syringes containing the autologous blood clot and hemostatic gelatin-based slurry are connected and vigorously agitated for another 50-60 passes to prepare a stable mixture.
- 7.
This mixture of hemostatic-gelatin based slurry, normal saline, iodinated contrast and autologous blood clot is then injected into the aneurysm sac slowly. Initially, 5 mL of the mixture is injected. The distribution of the mixture is checked with ultrasound or CT.
- 8.
The stasis of blood in the aneurysm sac is monitored with an ultrasound doppler or by CT or by the stoppage of the backflow of blood from the needle hub.
- 9.
While the needle is still in place, 2-0 nylon suture is introduced into the aneurysm sac. The sutures are introduced till the point resistance to passage is felt and at that point the suture is cut at the needle hub and a stylet is used to push the last strand into the aneurysm sac.
- 10.
Once stasis of blood in the pseudoaneurysm is confirmed, the needle is withdrawn while injecting small volume of the remaining mixture of gelatin-based hemostatic slurry and autologous blood clot to seal the needle puncture tract.
- 11.
The puncture site is monitored for any active hemorrhage and sterile dressing is applied at the puncture site.
Case 1
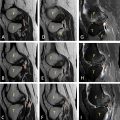
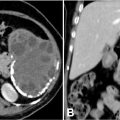
A 49-year-old man, already diagnosed with severe acute pancreatitis about a week back, now presented with profuse sweating and giddiness while trying to get up from the bed. His general look was that of profound pallor and his extremities were cold and clammy on initial examination. His pulse rate was 120 beats per minute with a noninvasive blood pressure recorded at 80/50 mmHg. His lab investigations were remarkable for a hemoglobin level of 5 g/dL, which showed a drastic drop from a previous recorded hemoglobin level of 10 g/dL. There was no history of bleeding from natural orifices. His abdomen was tender in the epigastric region, however, no new finding on abdominal examination was noted as compared to his previous records. With the clinical picture suggesting hypovolemic shock, immediate resuscitation was started, and 2 units of packed red blood cells were transfused. While the resuscitation was being carried out, a triple phase abdominal CT scan was done with the strong suspicion of a potential intra-abdominal hemorrhage. Contrast enhanced CT scan ( Fig. 1 ) sowed a large pseudoaneurysm of size ∼ 7.3 × 5.8 × 5.2 cm in the lesser sac, which appeared partially thrombosed. A part of the pseudoaneurysm towards the splenic hilum showed patent lumen of size ∼ 4.5 × 3.8 × 3.2 cm. The neck of the pseudoaneurysm was noted in relation to one of the distal hilar branches of the splenic artery. In view of the location of the aneurysm, the clinical condition of the patient and the financial considerations, it was decided to proceed with percutaneous direct embolization of the pseudoaneurysm. The procedural steps were carried out as per SAMEER technique under doppler ultrasound guidance ( Fig. 2 ), and complete hemostasis within the aneurysm sac was noted after a procedure time of ∼ 20 minutes. The patient improved clinically with resuscitation measures and did not receive any further blood transfusions. The aneurysm showed complete occlusion and no signs of recanalization at a follow-up doppler ultrasound at 3 weeks ( Fig. 2 ). His hemoglobin level was recorded at 9.4 g/dL and vitals were stable at 3 weeks follow-up after embolization.