Staging Breast Cancer
Although breast cancers appear to begin in the ductal or lobular epithelium, there is a wide variation in their growth and development. It is difficult to accurately predict the course of the disease for the individual woman. There may be exceptions, but for the most part these tumors grow first within the duct system. As they enlarge they develop the ability, probably through mutation or further chromosomal damage, to break out of the duct and infiltrate into the periductal stroma and gain access to the lymphatics and vascular structures, ultimately spreading to axillary and other lymph nodes and to distant organs.
It is becoming increasingly clear that each tumor is unique in its DNA pattern and with regard to the interaction of the tumor with the host. Efforts are under way to identify the specific traits of each tumor, with the goal of tailoring therapy for each woman based on the unique characteristics of her tumor and her body’s response to it. However, until this becomes possible, our understanding of breast cancer has come from looking at large numbers of women and the response of groups of women to therapeutic approaches. This grouping of what appear to be similar individuals is based on staging.
Staging uses definable tumor characteristics that have, over the years, provided the best information for trying to establish the prognosis for the individual and as an aid for determining which therapy might provide the best response. Although a crude measure, the labeling of a cancer permits a more accurate prognostication of its course and is a key factor in the testing of new approaches to treatment and the selection of treatment options. By allowing the comparison of tumors with similar characteristics, staging allows detection and therapeutic interventions to be compared.
Factors that crudely predict eventual outcome have been identified. The hope is to make this prognostication increasingly accurate, but for now a fairly simple system has been devised, and radiologists should be familiar with it. Staging is still very crude and does not yet take into account the features of each individual tumor and characteristics of the patient, but it provides an important frame of reference.
A cancer can be staged based on its clinical presentation and/or its pathologic features. The pathologic characteristics are usually the more accurate, reproducible, and predictive and are the features upon which most therapeutic decisions are based. Breast cancer staging recognizes three general levels of tumor involvement, loosely categorized by the locations of the growths:
Local: This pertains to cancer growth in the breast itself.
Regional: This pertains to cancer growth in the tissues contiguous to the breast (axilla, around the clavicle, and parasternal).
Distant: This pertains to metastatic tumor beyond the breast and its regional environment.
The most important prognostic features remain the size of the cancer (1), which is generally defined as its largest diameter (spiculations not included), and the axillary nodal status. The histologic grade of the tumor is also an important prognostic factor (2).
Major treatment decisions depend on whether the tumor is confined to the duct (intraductal cancer/ductal carcinoma in situ) or whether it is invasive, its size, and whether tumor has entered the lymphatics or blood vessels or is evident outside the breast in regional lymph nodes or other metastatic sites. Although in some women the presence of tumor in the lymphatics can become a direct problem (e.g., involvement of the brachial plexus), lymphatic involvement is an indication of the tumor’s biology and establishes its capacity to exist in organs outside the breast.
The primary reason for evaluating the axillary lymph nodes is for prognostication and as an aid in treatment selection, especially the decision to employ adjuvant chemotherapy or hormonal therapy. The demonstration that some women who are axillary lymph node negative may still benefit from adjuvant therapies has somewhat reduced the importance of assessing the axillary nodes (3), but most practitioners still prefer knowing the status of the axillary nodes as a measure of the tumor’s aggressivity.
Over the past several years the standard axillary dissection has led to a less morbid sampling of the lymph nodes guided by identifying the lymph nodes that appear to be the first draining nodes in the chain that drains the breast (sentinel node biopsy). Sentinel node biopsy has replaced axillary dissection in most major centers. These lymph nodes are predictive of the rest of the nodes in the axilla 95% of the time (4). By not having to do a full axillary dissection on every breast cancer patient, the morbidity of
the axillary surgery (e.g., arm edema) has been reduced. Staging has many uses. It may even be useful in determining whether axillary nodal dissection is needed (5).
the axillary surgery (e.g., arm edema) has been reduced. Staging has many uses. It may even be useful in determining whether axillary nodal dissection is needed (5).
Investigators have tried to subcategorize in situ and invasive cancers to permit more accurate prognostication. Barth et al (6), in an analysis of 918 patients with T1 breast cancers (2 cm or less), found that 23% had positive axillary lymph nodes. They found, however, that lymph node positivity varied with certain factors. The strongest predictors of axillary nodal involvement were the presence of intramammary lymphatic or vascular invasion, a palpable tumor, and a high nuclear grade. The likelihood of positivity increased with tumor size. They found that if a tumor was nonpalpable, T1a or T1b without lymph or vascular invasion, and not high grade, the incidence of positive nodes was only 3%. If, however, the lesion was palpable, had lymph or vascular invasion, was high grade, and was 1 to 2 cm in size, lymph node positivity increased to 49%. Women with a nonpalpable tumor of low or intermediate grade that is 1 cm or smaller with no lymph vessel or vascular invasion have such a low risk of nodal involvement that axillary dissection may not be required.
The American Joint Committee on Cancer (AJCC) uses the TNM classification system to define stages. The size of the primary tumor determines the T, the status of the regional lymph nodes the N, and the presence or absence of distant metastases the M.
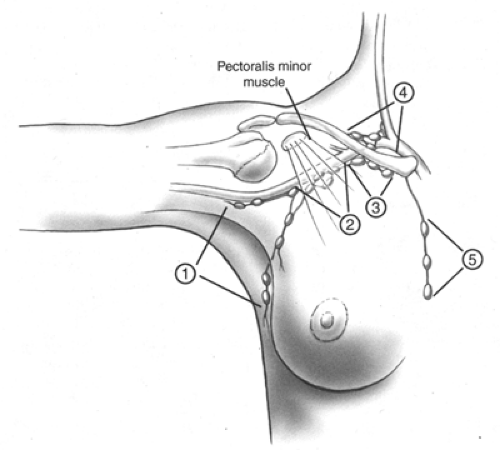
As noted above, the primary site is the breast itself, along with its skin and chest wall attachments. Regional lymph nodes are those in the axilla. Although lymphatic drainage of the breast also includes the internal mammary chain as well as nodes along channels through the pectoralis muscles, the nodes most commonly affected and clinically accessible are those in the axilla, and it is primarily these that are studied for staging purposes. Axillary nodes have been subdivided into levels (Fig. 6-1). Level I nodes are those that are in the axilla lateral to the pectoralis major muscle. Level II lymph nodes are high in the apex of the axilla, between the pectoralis major and minor muscles, and level III nodes are infraclavicular. Tumor in supraclavicular nodes or involvement of any other lymph node group, including internal mammary nodes, is considered distant metastasis.
Cancers can be staged at the clinical level by visual inspection, palpation, and mammography, with tumor confirmation by biopsy. Most treatment protocols, however, use pathologic staging. Pathologic staging includes the gross assessment of the size of the excised tumor as well as histologic evaluation of the tumor, its type (if it can be classified), the grade of the cells (grade 1 is well differentiated and grade 3 is poorly differentiated) and the remainder of the breast (if a mastectomy has been performed), and the microscopic evaluation of the axillary lymph nodes removed at surgery. Studies suggest that at least 10 axillary lymph nodes should be excised from level I and perhaps level II to ensure an accurate evaluation of regional involvement (7). Staging now allows for categorization as to how the axillary nodes were assessed to account for the widespread adoption of sentinel node biopsy. Even though adjuvant therapy is to be used, regardless of the node status, the presence or absence of tumor in axillary nodes is important for prognostication and future analysis of therapeutic decisions.
The actual number of involved nodes appears to directly affect prognosis. Prognostically, the absence of nodal involvement offers the greatest chance of cure, although as many as 30% of women who are lymph node negative develop recurrent breast cancer. Women with one to three nodes involved have a diminished survival expectation but do better than women with four or more involved nodes (8). Some studies indicate that infiltration of tumor from the node into the surrounding tissue is an additional adverse prognostic factor.
Women who are lymph node positive at diagnosis can benefit from adjuvant chemotherapy or hormonal therapy (9). Women with large and more aggressive tumors may also benefit from adjuvant therapy even if they are node negative.
Most of the available data are based on a rather crude analysis of lymph nodes. The node is bivalved, and several slices are shaved from each surface. The percentage of women with positive nodes increases as more thorough investigation is carried out. Staging and the conclusions based on staging are evolving as even more sensitive tests using immunohistochemical techniques, or even polymerase chain reaction DNA studies, can detect even a few cancer cells in the lymph nodes. Because there are fewer nodes to evaluate, the change to sentinel node biopsy has led to a more detailed evaluation of the lymph nodes, and even a few cancer cells can be identified in these nodes. Their importance remains to be determined, but the updated staging system allows for distinguishing how the lymph nodes were evaluated so that over time it is hoped that the importance of this more detailed evaluation can be determined.
Although staging initially appears complex, it basically involves the size of the tumor and the presence or absence of tumor in axillary nodes or distant sites. The stage of the tumor depends on the combinations of these factors and can be simplified as follows:
Stage I cancers are less than or equal to 2 cm in diameter and have no evidence of axillary or distant metastases.
Stage II cancers are larger than 2 cm but not larger than 5 cm in diameter, or they are tumors with associated involvement of the axillary nodes but without distant metastatic spread.
Stage III cancers are tumors of any size in which the lymph nodes are fixed together in a matted axillary mass (IIIa), or tumors in which there is extension to the chest wall, or there is skin involvement, or both (IIIb).
Stage IV cancers include any tumor with associated distant metastases.
The full classification system is included in the appendix to this chapter.
References
1. Michaelson JS, Silverstein M, Sgroi D, et al. The effect of tumor size and lymph node status on breast carcinoma lethality. Cancer 2003;98:2133–2143.
2. Henson DE, Ries L, Freedman LS, et al. Relationship among outcome, stage of disease, and histologic grade for 22,616 cases of breast cancer. The basis for a prognostic index. Cancer 1991;68:2142–2149.
3. Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. Lancet 1992;339:1–15,71–85.
4. Harlow SP, Krag DN. Sentinel lymph node biopsy in breast cancer. Breast Dis 2001;12:43–55.
5. Taneja C, Cady B. Decreasing role of lymphatic system surgery in surgical oncology. J Surg Oncol 2005;89:61–66.
6. Barth A, Craig PH, Silverstein MJ. Predictors of axillary lymph node metastases in patients with T1 breast carcinoma. Cancer 1997;79:1918–1922.
7. Bonadonna G, Zambetti M, Valagussa P. Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes: ten-year results. JAMA 1995;273:542–547.
8. Winchester DP, Cox JD. Standards for breast-conservation treatment. CA Cancer J Clin 1992;42:134–162.
9. Consensus Development Panel. Adjuvant chemotherapy for breast cancer: consensus conference. JAMA 1985;254:3461.
C50.0 Nipple
C50.1 Central portion of breast
C50.2 Upper inner quadrant of breast
C50.3 Lower inner quadrant of breast
C50.4 Upper outer quadrant of breast
C50.5 Lower outer quadrant of breast
C50.6 Axillary tail of breast
C50.8 Overlapping lesion of breast
C50.9 Breast, NOS
| SUMMARY OF CHANGES |
|
Introduction
This staging system for carcinoma of the breast applies to infiltrating (including microinvasive) and in situ carcinomas. Microscopic confirmation of the diagnosis is mandatory, and the histologic type and grade of carcinoma should be recorded.
Anatomy
Primary Site
The mammary gland, situated on the anterior chest wall, is composed of glandular tissue with a dense fibrous stroma. The glandular tissue consists of lobules that group
together into 15–25 lobes arranged approximately in a spoke-like pattern. Multiple major and minor ducts connect the milk-secreting lobular units to the nipple. Small milk ducts course throughout the breast, converging into larger collecting ducts that open into the lactiferous sinus at the base of the nipple. Most cancers form initially in the terminal duct lobular units of the breast. Glandular tissue is more abundant in the upper outer portion of the breast; as a result, half of all breast cancers occur in this area.
together into 15–25 lobes arranged approximately in a spoke-like pattern. Multiple major and minor ducts connect the milk-secreting lobular units to the nipple. Small milk ducts course throughout the breast, converging into larger collecting ducts that open into the lactiferous sinus at the base of the nipple. Most cancers form initially in the terminal duct lobular units of the breast. Glandular tissue is more abundant in the upper outer portion of the breast; as a result, half of all breast cancers occur in this area.
Chest Wall
The chest wall includes ribs, intercostal muscles, and serratus anterior muscle, but not the pectoral muscles.
Regional Lymph Nodes
The breast lymphatics drain by way of three major routes: axillary, transpectoral, and internal mammary. Intramammary lymph nodes are coded as axillary lymph nodes for staging purposes. Supraclavicular lymph nodes are classified as regional lymph nodes for staging purposes. Metastasis to any other lymph node, including cervical or contralateral internal mammary lymph nodes, is classified as distant (M1) (Fig. 6-1).
The regional lymph nodes are as follows:
Axillary (ipsilateral): interpectoral (Rotter’s) nodes and lymph nodes along the axillary vein and its tributaries that may be (but are not required to be) divided into the following levels:
Level I (low-axilla): lymph nodes lateral to the lateral border of pectoralis minor muscle.
Level II (mid-axilla); lymph nodes between the medial and lateral borders of the pectoralis minor muscle and the interpectoral (Rotter’s) lymph nodes,
Level III (apical axilla): lymph nodes medial to the medial margin of the pectoralis minor muscle, including those designated as apical.
Internal mammary (ipsilateral): lymph nodes in the intercostal spaces along the edge of the sternum in the endothoracic fascia.
Supraclavicular: lymph nodes in the supraclavicular fossa, a triangle defined by the omohyoid muscle and tendon (lateral and superior border), the internal jugular vein (medial border), and the clavicle and subclavian vein (lower border). Adjacent lymph nodes outside of this triangle are considered to be lower cervical nodes (M1).
Metastatic Sites
Tumor cells may be disseminated by either the lymphatic or the blood vascular system. The four major sites of involvement are bone, lung, brain, and liver, but tumor cells are also capable of metastasizing to many other sites.
Rules For Classification
Clinical Staging
Clinical staging includes physical examination, with careful inspection and palpation of the skin, mammary gland, and lymph nodes (axillary, supraclavicular, and cervical), imaging, and pathologic examination of the breast or other tissues as appropriate to establish the diagnosis of breast carcinoma. The extent of tissue examined pathologically for clinical staging is not so great as that required for pathologic staging (see Pathologic Staging below). Imaging findings are considered elements of staging if they are collected within 4 months of diagnosis in the absence of disease progression or through completion of surgery(ies), whichever is longer. Such imaging findings would include the size of the primary tumor and of chest wall invasion, and the presence or absence of regional or distant metastasis. Imaging findings and surgical findings obtained after a patient has been treated with neoadjuvant chemotherapy, hormonal therapy, immunotherapy, or radiation therapy are not considered elements of initial staging.
Pathologic Staging
Pathologic staging includes all data used for clinical staging, plus data from surgical exploration and resection as well as pathologic examination of the primary carcinoma, regional lymph nodes, and metastatic sites (if applicable), including not less than excision of the primary carcinoma with no macroscopic tumor in any margin of resection by pathologic examination. A cancer can be classified pT for pathologic stage grouping if there is only microscopic, but not macroscopic, involvement at the margin. If there is tumor in the margin of resection by macroscopic examination, the cancer is coded pTX because the total extent of the primary tumor cannot be assessed. If the primary tumor is invasive and not only microinvasive, resection of at least the low axillary lymph nodes (Level I)—that is, those lymph nodes located lateral to the lateral border of the pectoralis minor muscle—should be performed for pathologic (pN) classification. Such a resection will ordinarily include six or more lymph nodes. Alternatively, one or more sentinel lymph nodes may be resected and examined for pathologic classification. Certain histologic tumor types (pure tubular carcinoma <1 cm, pure mucinous carcinoma <1 cm, and microinvasive carcinoma) have a very low incidence of axillary lymph node metastasis and do not usually require an axillary lymph node dissection. Cancerous nodules in the axillary fat adjacent to the breast, without histologic evidence of residual lymph node tissue, are classified as regional lymph node metastases (N). Pathologic stage grouping includes any of the following combinations of pathologic and clinical classifications: pT pN pM, or pT pN cM, or cT cN pM. If surgery occurs after the patient has received neoadjuvant chemotherapy, hormonal therapy, immunotherapy, or radiation therapy, the prefix “y” should be used with the TNM classification, e.g., ypTNM.
TNM Classification
Primary Tumor (T)
Determining Tumor Size
The clinical measurement used for classifying the primary tumor (T) is the one judged to be most accurate for that particular case (that is, physical examination or imaging such as mammography or ultrasound). The pathologic tumor size for the T classification is a measurement of only the invasive component. For example, if there is a 4.0-cm intraductal component and a 0.3-cm invasive component, the tumor is classified T1a. The size of the primary tumor is measured for T classification before any tissue is removed for special studies, such as for estrogen receptors. In patients who have received multiple core biopsies, measuring only the residual lesion may result in significantly underclassifying the T component and thus understaging the tumor. In such cases, original tumor size should be reconstructed on the basis of a combination of imaging and all histologic findings.
Tis Classification
Carcinoma in situ, with no evidence of an invasive component, is classified as Tis, with a subclassification indicating type. Cases of ductal carcinoma in situ and cases with both (ductal carcinoma in situ and lobular carcinoma in situ are classified Tis (DCIS). Lobular carcinoma in situ is increasingly defined as a risk factor for subsequent breast cancer, although there is some evidence that it may occasionally be a precursor of invasive lobular carcinoma. For example, this may be the case with LCIS with more atypical cytology (pleomorphic) as well as more extensive and locally distorting examples of well-developed LCIS (1). Regardless of this controversy, LCIS is reported as a malignancy by national database registrars and should be designated as such in this classification system—e.g., Tis (LCIS), Paget’s disease of the nipple without an associated tumor mass (clinical) or invasive carcinoma (pathologic) is classified Tis (Paget’s), Paget’s disease with a demonstrable mass (clinical) anywhere within that breast or an invasive component (pathologic) is classified according to the size of the tumor mass or invasive component.
Microinvasion of Breast Carcinoma
Microinvasion is the extension of cancer cells beyond the basement membrane into the adjacent tissues with no focus more than 0.1 cm in greatest dimension. When there are multiple foci of microinvasion, the size of only the largest focus is used to classify the microinvasion. (Do not use the sum of all the individual foci.) The presence of multiple foci of microinvasion should be noted and/or quantified, as it is with multiple larger invasive carcinomas.
Multiple Simultaneous Ipsilateral Primary Carcinomas
The following guidelines are used in classifying multiple simultaneous ipsilateral primary (infiltrating, macroscopically measurable) carcinomas. These criteria do not apply to one macroscopic carcinoma associated with multiple separate microscopic foci. Most conservatively, tumors are defined as arising independently only if they occur in different quadrants of the breast.
Use the largest primary carcinoma to designate T classification. Do not assign a separate T classification for the smaller tumor(s).
Enter into the record that this is a case of multiple simultaneous ipsilateral primary carcinomas. The outcome of such cases should be analyzed separately.
Simultaneous Bilateral Breast Carcinomas
Each carcinoma is staged as a separate primary carcinoma in a separate organ,
Inflammatory Carcinoma
Inflammatory carcinoma is a clinicopathologic entity characterized by diffuse erythema and edema (peau d’orange) of the breast, often without an underlying palpable mass. These clinical findings should involve the majority of the skin of the breast. Classically, the skin changes arise quickly in the affected breast. Thus the term inflammatory carcinoma should not be applied to a patient with neglected locally advanced cancer of the breast presenting late in the course of her disease. On imaging, there may be a detectable mass and characteristic thickening of the skin over the breast. This clinical presentation is due to tumor emboli within dermal lymphatics, which may or may not be apparent on skin biopsy. The tumor of inflammatory carcinoma is classified T4d. It is important to remember that inflammatory carcinoma is primarily a clinical diagnosis. Involvement of the dermal lymphatics alone does not indicate inflammatory carcinoma in the absence of clinical findings. In addition to the clinical picture, however, a biopsy is still necessary to demonstrate cancer either within the dermal lymphatics or in the breast parenchyma itself.
Skin of Breast
Dimpling of the skin, nipple retraction, or any other skin change except those described under T4b and T4d may occur in T1, T2, or T3 without changing the classification.
Regional Lymph Nodes (N)
Macrometastasis
Cases in which regional lymph nodes cannot be assessed (previously removed or not removed for pathologic examination) are designated NX or pNX. Cases in which no regional lymph node metastasis is detected are designated NO or pNO.
In patients who are clinically node-positive, N1 designates metastasis to one or more movable ipsilateral axillary lymph nodes, N2a designates metastasis to axillary lymph nodes that are fixed to each other (matted) or to other structures, and N3a indicates metastasis to ipsilateral infraclavicular lymph nodes. Metastasis to the ipsilateral internal mammary nodes are designated as N2b when they are detected by imaging studies (including CT scan and ultrasound, but excluding lymphoscintigraphy) or by clinical examination and when they do not occur in conjunction with metastasis to the axillary lymph nodes. Metastases to the ipsilateral internal mammary nodes are designated as N3b when they are detected by imaging studies or by clinical examination and when they occur in conjunction with metastasis to the axillary lymph nodes. Metastasis to the ipsilateral supraclavicular lymph nodes are designated as N3c regardless of the presence or absence of axillary or internal mammary nodal involvement.
In patients who are pathologically node-positive with one or more tumor deposits greater than 2 mm, cases with 1 to 3 positive axillary lymph nodes are classified pN1a, cases with 4 to 9 positive axillary lymph nodes are classified pN2a, and cases with 10 or more positive axillary lymph nodes are classified pN3a. Cases with histologically confirmed metastasis to the internal mammary nodes, detected by sentinel lymph node dissection but not by imaging studies (excluding lymphoscintigraphy) or clinical examination, are classified as pN1b if occurring in the absence of metastasis to the axillary lymph nodes and as pN1c if occurring in the presence of metastases to 1 to 3 axillary lymph nodes. (If 4 or more axillary lymph nodes are involved, the classification pN3b is used.) Clinical involvement, with histologic confirmation of the internal mammary nodes by imaging studies (excluding lymphoscintigraphy) in the. absence or presence of axillary nodal metastases are classified as pN2b and pN3b, respectively. Histologic evidence of metastasis in ipsilateral supraclavicular lymph node(s) is classified as pN3c. A classification of pN3, regardless of primary tumor size or grade, is classified as Stage IIIC. A case in which the classification is based only on sentinel lymph node dissection is given the additional designation (sn) for “sentinel node”—for example, pN1 (sn). For a case in which an initial classification is based on a sentinel lymph node dissection but a standard axillary lymph node dissection is subsequently performed, the classification is based on the total results of the axillary lymph node dissection (that is, including the sentinel node).
Isolated Tumor Cells and Micrometastases
Isolated tumor cells (ITCs) are defined as single cells or small clusters of cells not greater than 0.2 mm in largest dimension, usually with no histologic evidence of malignant activity (such as proliferation or stromal reaction). If an additional immunohistochemical examination was made for ITCs in a patient with histologically negative
lymph nodes, the regional lymph nodes should be designated as pN0(i-) or pN0(i+), as appropriate.
lymph nodes, the regional lymph nodes should be designated as pN0(i-) or pN0(i+), as appropriate.
Micrometastases are defined as tumor deposits greater than 0.2 mm but not greater than 2.0 mm in largest dimension that may have histologic evidence of malignant activity (such as proliferation or stromal reaction). Cases in which only micrometastases are detected (none greater than 2 mm) are classified pN1mi. The classification is designated as (i+) for “immunohistochemical” if micrometastasis was detected only by IHC [e.g., pN1mi (i+)].
If histologically and immunohistochemically negative lymph nodes are examined for evidence of metastasis using molecular methods [reverse transcriptase–polymerase chain reaction (RT-PCR)], the regional lymph nodes are classified as pN0(mol-) or pN0(mol+), as appropriate.
Distant Metastasis (M)
Cases where distant metastasis cannot be assessed are designated MX, cases in which there is no distant metastasis are designated M0, and cases in which one or more distant metastases are identified are designated M1. A negative clinical history and examination are sufficient to designate a case as M0; extensive imaging or other testing is not required. Note that positive supraclavicular lymph nodes are now classified as N3 rather than M1.
Definition of TNM
Primary Tumor (T)
Definitions for classifying the primary tumor (T) are the same for clinical and for pathologic classification. If the measurement is made by physical examination, the examiner will use the major headings (T1, T2, or T3). If other measurements, such as mammographic or pathologic measurements, are used, the subsets of T1 can be used. Tumors should be measured to the nearest 0.1 cm increment.
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| Tis (DCIS) | Ductal carcinoma in situ |
| Tis (LCIS) | Lobular carcinoma in situ |
| Tis (Paget’s) | Paget’s disease of the nipple with no tumor |
Note: Paget’s disease associated with a tumor is classified according to the size of the tumor.
| T1 | Tumor 2 cm or less in greatest dimension |
| T1mic | Microinvasion 0.1 cm or less in greatest dimension |
| T1a | Tumor more than 0.1 cm but not more than 0.5 cm in greatest dimension |
| T1b | Tumor more than 0.5 cm but not more than 1 cm in greatest dimension |
| T1c | Tumor more than 1 cm but not more than 2 cm in greatest dimension |
| T2 | Tumor more than 2 cm but not more than 5 cm in greatest dimension |
| T3 | Tumor more than 5 cm in greatest dimension |
| T4 | Tumor of any size with direct extension to (a) chest wall or (b) skin, only as described below |
| T4a | Extension to chest wall, not including pectoralis muscle |
| T4b | Edema (including peau d’orange) or ulceration of the skin of the breast, or satellite skin nodules confined to the same breast |
| T4c | Both T4a and T4b |
| T4d | Inflammatory carcinoma |
Regional Lymph Nodes (N)
| Clinical | |
| NX | Regional lymph nodes cannot be assessed (e.g., previously removed) |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis to movable ipsilateral axillary lymph node(s) |
| N2 | Metastases in ipsilateral axillary lymph nodes fixed or matted, or in clinically apparent* ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastasis |
| N2a | Metastasis in ipsilateral axillary lymph nodes fixed to one another (matted) or to other structures |
| N2b | Metastasis only in clinically apparent* ipsilateral internal mammary nodes and in the absence of clinically evident axillary lymph node metastasis |
| N3 | Metastasis in ipsilateral infraclavicular lymph node(s) with or without axillary lymph node involvement, or in clinically apparent* ipsilateral internal mammary lymph node(s) and in the presence of clinically evident axillary lymph node metastasis; or metastasis in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement |
| N3a | Metastasis in ipsilateral infraclavicular lymph node(s) |
| N3b | Metastasis in ipsilateral internal mammary lymph node(s) and axillary lymph node(s) |
| N3c | Metastasis in ipsilateral supraclavicular lymph node(s) |
| *Clinically apparent is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination or grossly visible pathologically. | |
| Pathologic (pN)a | |
| NX | Regional lymph nodes cannot be assessed (e.g., previously removed, or not removed for pathologic study) |
| pN0 | No regional lymph node metastasis histologically, no additional examination for isolated tumor cells (ITC) |
| aClassification is based on axillary lymph node dissection with or without sentinel lymph node dissection. Classification based solely on sentinel lymph node dissection without subsequent axillary lymph node dissection is designated (sn) for “sentinel node,” e.g., pN0(i+) (sn). | |
Note: Isolated tumor cells (ITC) are defined as single tumor cells or small cell clusters not greater than 0.2 mm, usually detected only by immunohistochemical (IHC) or molecular methods but which may be verified on H&E stains. ITCs do not usually show evidence of malignant activity, e.g., proliferation or stromal reaction.
| pN0(i-) | No regional lymph node metastasis histologically, negative IHC |
| pN0(i+) | No regional lymph node metastasis histologically, positive IHC, no IHC cluster greater than 0.2 mm |
| pN0(mol-) | No regional lymph node metastasis histologically, negative molecular findings (RT-PCR)b |
| pN0(mol+) | No regional lymph node metastasis histologically, positive molecular findings (RT-PCR)b |
| pN1 | Metastasis in 1 to 3 axillary lymph nodes, and/or in internal mammary nodes with microscopic disease detected by sentinel lymph node dissection but not clinically apparent** |
| pN1mi | Micrometastasis (greater than 0.2 mm, none greater than 2.0 mm) |
| pN1a | Metastasis in 1 to 3 axillary lymph nodes |
| pN1b |