Abstract
We report the first successful coil embolization using a gadolinium contrast agent (GCA) of 3 pulmonary arteriovenous malformations (PAVMs) in a patient with severe iodine allergy. The patient was a female in her 40s with hereditary hemorrhagic telangiectasia. The 3 PAVMs had sacs with diameters of up to 20 mm and pulmonary feeding arteries with diameters of up to 5 mm. The PAVMs were located in segment (S) 8 and S9 of the right lung, and S8 of the left lung. Embolization was planned after administration of steroids and antihistamines. However, the procedure was stopped because preoperative pulmonary arteriography using an iodine contrast agent caused pharyngeal itching, coughing, and decreased percutaneous oxygen saturation. Because surgical resection is highly invasive, embolization using a GCA was selected as an alternative treatment strategy. After obtaining approval from our hospital’s ethical review board, embolization with a GCA was planned. Embolization was performed in 3 separate sessions. The catheter’s position was monitored and adjusted under angiography with manual injection of a GCA and low-dose computed tomography (CT) using an interventional radiology-CT system. The GCA was visible on angiography and coil embolization of the PAVM sacs and feeding arteries was performed. A small amount of GCA was used (7‒9 mL). The patient experienced no allergic reactions during the procedure. Although there are many reports of endovascular treatment using GCA for dialysis shunt stenosis and cerebrovascular disease in patients with iodine allergy, this is the first report describing the use of GCA during PAVM embolization.
Introduction
Pulmonary arteriovenous malformations (PAVMs) cause a variety of complications, such as cerebral infarction, brain abscess, and hypoxemia. Complications of the central nervous system occur in about 40% of patients with PAVMs [ ]. In addition, about 70% of patients with PAVMs have hereditary hemorrhagic telangiectasia (HHT) [ ]. First-line treatment of PAVMs is transpulmonary arterial embolization (TAE). Iodine contrast agents (ICAs) are commonly used during TAE. However, ICAs cause adverse events in 3.13% of patients and severe adverse events, such as anaphylactic shock, in 0.04% of patients [ ]. By comparison, gadolinium contrast agents (GCAs) have a lower risk (2.4%) of adverse events than ICAs [ ], and serious adverse events occur in 0.001%-0.01% of patients [ ]. In general, ICAs should not be administered in patients with severe allergies to iodine. In such patients, carbon dioxide (CO2) or GCAs have been used as an alternative contrast agent in patients undergoing interventional radiology (IVR) [ ]. However, CO2 may cause air embolism through shunt vessels, and it is contraindicated in patients with PAVMs. Although GCAs have been used in balloon-occluded retrograde transvenous obliteration [ ] and embolization of direct carotid cavernous fistulas [ ], there are no reports describing the use of GCAs in patients undergoing embolization of PAVMs. We report the first case of successful use of a GCA for coil embolization of 3 PAVMs in a patient with an iodine allergy.
Case report
This procedure was approved by the ethical review board of our institution. The patient provided informed consent for publication of this case report.
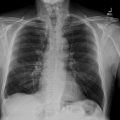
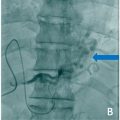
A female patient in her 40s visited another hospital due to difficulty breathing and nasal bleeding. A noncontrast enhanced computed tomography (CT) scan of the chest revealed 3 PAVMs. She was referred to our hospital’s thoracic surgery department. However, surgery was considered to be too invasive and difficult. For this reason, she was transferred to our department for TAE. HHT was suspected based on her family history of repeated nasal bleeding in first-degree relatives. Genetic testing revealed a mutation in the Endoglin gene and she was diagnosed with HHT. A plain chest X-ray showed 3 nodular shadows in the bilateral lower lung areas ( Fig. 1 ). ICA-enhanced CT showed 3 simple PAVMs in segment (S) 8 and S9 of the right lung, and S8 of the left lung ( Fig. 2 A‒C). The diameters of the feeding pulmonary arteries and sacs of the 3 PAVMs were 3.3 and 11 mm, 3.5 and 9 mm, and 3.0 and 10 mm.


During the enhanced CT using an ICA (Iopromide 300; Bayer, Nordrhein-Westfalen, Germany), she developed a wheal and itchy skin, which led to diagnosis of an iodine allergy. Her vital signs were as follows: a consciousness state (Glasgow coma scale) of E4V5M6, oxygen saturation of 94% (without oxygen administration), heart rate of 80 beats/min, clear breathing sounds, no sounds indicative of heart murmur, and electrocardiogram indicated sinus rhythm without arrhythmia. A blood test showed a red blood cell count of 4 million cells/µL, hemoglobin level of 12.1 g/dL, platelet count of 264,000 cells/µL, creatinine level of 0.69 mg/dL, and an estimated glomerular filtration rate (eGFR) of 71.3 mL/min/1.73 m 2 .
Percutaneous TAE of the PAVMs
We planned to perform TAE using an ICA under local anesthesia in our angiography room, which is equipped with an IVR-CT system (Aquilion One, Alphenix, Canon, Tochigi, Japan). Antihistamines and steroids were administered to the patient to prevent an allergic reaction. A 7 Fr sheath (Radifocus introducer II H, Terumo, Tokyo, Japan) was inserted into the right femoral vein under ultrasound guidance. A 7 Fr guiding catheter (Coolnand, Medikit, Tokyo, Japan) and a 5 Fr catheter (VTA-5M catheter, Medikit) were then inserted for angiography of the right lower lobe branch (A8) of the pulmonary artery with ICA (Iopamidol 300, Fuji Pharma, Tokyo, Japan) ( Fig. 3 ). Immediately after ICA administration, the patient developed wheals, pharyngeal itching, cough, a decrease in oxygen saturation (from 94% to 90%), and a decrease in systolic blood pressure (from 140 to 110 mmHg). Although her vital signs improved after administration of oxygen, antihistamines, and steroids, the procedure was stopped. Therefore, TAE using a GCA as an alternative contrast agent was planned.

TAE of the PAVMs located in the right lung S8, left lung S8, and right lung S9 were performed in 3 sessions to reduce the amount of GCA used per session. In each procedure, we used gadoteridol (ProHance, Bracco, Milan, Italy) as the GCA. We also performed low-dose CT using the IVR-CT system to confirm the catheter tip’s position, while advancing the catheter to the target in each procedure. The TAE procedures were performed in the angiography room under local anesthesia after premedication with antihistamines and steroids. A 7 Fr sheath was inserted via the right femoral vein under ultrasound guidance. A 7 Fr guiding catheter (Coolnand, Medikit) and a 5 Fr catheter (VTE catheter, Medikit) were inserted into the feeding pulmonary arteries of the PAVMs. A microcatheter (Carry Slim Leon, UTM, Aichi, Japan) was carefully advanced to the target using a 0.014-in. microguidewire (CHIKAI V, Asahi Intec, Aichi, Japan), while confirming the locations of the microcatheter’s tip and the target using low-dose CT. The PAVM in S8 of the left lung was detected by manual digital subtraction angiography (DSA) using GCA ( Fig. 4 A). A microcatheter was advanced into the pulmonary vein side of the sac. The sac and feeding artery were then embolized using 6 microcoils. Manual injection DSA using the GCA after embolization showed complete occlusion of the sac and feeding artery ( Fig. 4 B). The other PAVMs in S8 and S9 of the right lung were embolized with the same technique.