Superficial Soft Tissues Masses
Superficial soft tissue masses are common in clinical practice and the expanding availability of radiologic imaging has increased radiologists’ familiarity with these entities. Superficial soft tissue masses are quite diverse and include tumors, as well as many nonmesenchymal tumor-like lesions.
In the case of some masses, such as superficial lipomas, the imaging characteristics will enable a definitive diagnosis. However, the imaging features of many other superficial soft tissue lesions may be disappointingly nonspecific; therefore, the possible diagnostic considerations initially may seem overwhelming. In such cases, the use of a systematic approach can help narrow the differential diagnosis. Superficial soft tissue masses may be classified in one of the following general diagnostic categories: mesenchymal tumors, skin appendage lesions, nonmesenchymal tumors, other tumors and tumor-like lesions, and inflammatory lesions.
This chapter reflects our experience with superficial soft tissue masses. It is not intended as a comprehensive review or rigid classification system, but rather, as an overview, with emphases on lesions that are more common or relatively more common, and on lesions in which diagnoses may be suggested by magnetic resonance (MR) imaging features. We have included some lesions that extend beyond the deep fascia, as well as some that may affect both the superficial and deep soft tissues.
SKIN APPENDAGE LESIONS
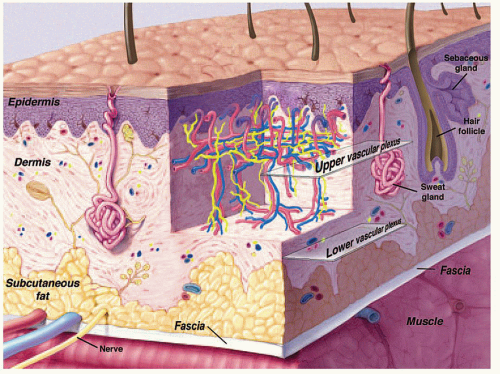
Skin appendage lesions originate in the epidermis and dermis (Fig. 12.1) (1). They are common and are typically subcategorized as proliferations of follicular lineage or eccrine-apocrine differentiation. The lesions are typically small and diagnosed clinically. Imaging is usually reserved for unusually large or atypical lesions; however, skin appendage lesions are not uncommonly identified as incidental imaging findings.
Epidermal Inclusion Cyst
The epidermal inclusion cyst is one of the most common, benign, dermatological lesions and the most common dermal epithelial cyst (2). Also known as infundibular cyst or inclusion cyst, it is a simple epithelial cyst lined with infundibular or epidermis-like cells that keratinize (3). The majority form as a result of progressive cystic ectasia of the infundibular (upper) portion of the hair follicle, as a result of mechanical obstruction, scarring, or inflammation (3). In this sense, most are retention cysts rather than inclusion cysts (3). The term sebaceous cyst is a misnomer and should be avoided, because these cysts are not of sebaceous differentiation
and their imaging characteristics vary according to their internal contents (1).
and their imaging characteristics vary according to their internal contents (1).
KEY CONCEPTS
Epidermal inclusion cysts may occur anywhere, but are most common in the head, neck, and trunk.
On MR imaging, the lesion appears as a small, welldefined, ovoid to lobulated, subcutaneous mass.
MR typically shows cyst-like signal intensity.
Increased signal intensity can be seen on T1-weighted images, due to increased proteinaceous cyst contents.
The character of the lesion varies with the contents of the cyst.
Lesions are adjacent to the dermis and will scallop the skin in about half of the cases.
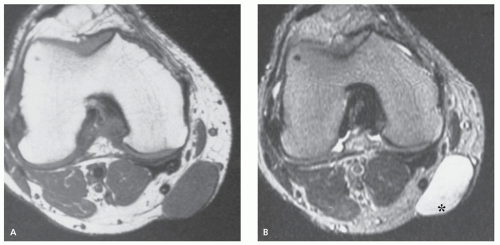
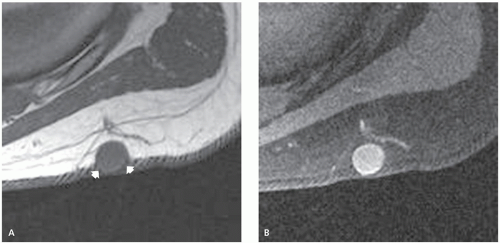
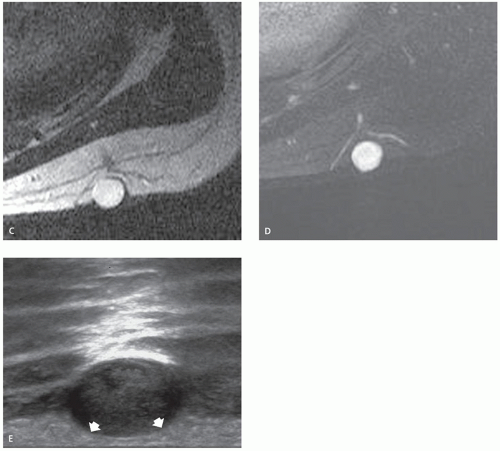
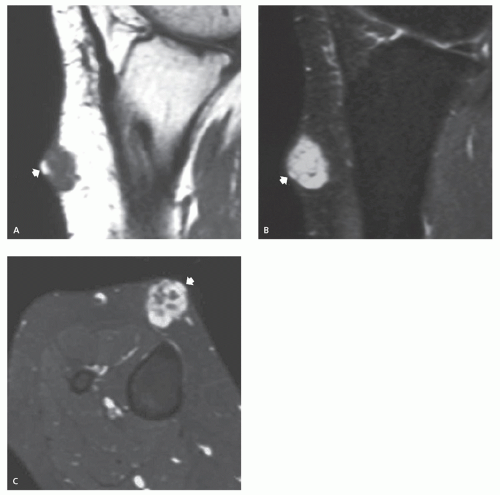
These lesions may occur anywhere, but they are most common in the head, neck, and trunk (3). They are typically small, subcutaneous or cutaneous nodules less than 5 cm in size (3). Cysts are filled with loosely packed lamellae of keratin and the walls of the cyst resemble follicular infundibular epithelium (3). Cyst walls may rupture, with secondary foreign body-type reaction, granulomatous reaction, granulation tissue, or abscess formation (3, 4). Secondary bacterial infection may also occur. Although these lesions are typically solitary, multiple lesions are reported (5). The clinical history is usually that of painless soft tissue mass, with variable growth, that may be present from months to years (6).
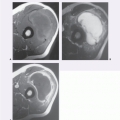
On MR imaging, the lesion appears as a small, welldefined, ovoid to lobulated, subcutaneous mass. The character of the lesion varies with the contents of the cyst, and large lesions may show dependent debris (Figs. 12.2 and 12.3) (6). MR imaging will typically show a cyst-like signal intensity, although increased signal intensity can be seen on T1-weighted images, due increased proteinaceous contents (7, 8). Mild heterogeneity is not uncommon, as is a signal intensity less than that of fat on nonfatsuppressed T2-weighted images. Following contrast administration, a thin rind of enhancement is seen (9). It has been our experience that lesions are typically adjacent to the dermis and will scallop the skin in about half of cases; a feature that is usually well seen on both MR imaging and ultrasound. At ultrasound, the cyst appears as a circumscribed mass of variable echogenicity, similar to or less than that of the adjacent fat, with prominent through transmission (Fig. 12.3).
Pilomatricoma
Pilomatricoma, originally known as calcifying epithelioma of Malherbe, was initially described by Malherbe and Chenantais in 1880 as a benign calcifying tumor thought to arise from skin appendages (10). The lesion was renamed pilomatixoma by Forbis and Helwig in 1961 (11), to avoid a connotation of malignancy and to emphasize that the lesion arises in the dermis, from primitive
cells that normally differentiate toward hair matrix cells (11, 12, 13). More recently, the lesion has been termed pilomatricoma (12).
cells that normally differentiate toward hair matrix cells (11, 12, 13). More recently, the lesion has been termed pilomatricoma (12).
KEY CONCEPTS
Pilomatricoma is also known as calcifying epithelioma of Malherbe.
The lesion arises in the dermis, from cells that normally differentiate toward hair matrix cells.
The face, neck, and upper extremities are most commonly affected.
Lesions are small, averaging about 1 cm in diameter, and typically less than 3 cm.
Calcification is seen on radiographs in about 50% of cases, and on CT in about 80%.
Lesions show an intermediate signal intensity on MR imaging; associated inflammatory change is seen in about half of the cases.
Most patients are young, with a peak incidence between 10 and 15 years of age (10). Approximately 40% occur in children younger than 10 years (13, 14). Although pilomatricoma accounts for less than 1% of skin tumors, it is the most common solid cutaneous tumor in patients 20 years of age and younger (15) and the most commonly excised superficial mass in children after epidermal inclusion cyst (16). There is a secondary peak occurring in mature adults (aged 41 to 70 years) (12). The face, neck, and upper extremities are most commonly affected (10, 14, 17, 18), with about 50% to 75% of all lesions occurring in the head and neck (12, 16, 18). Tumors are small, painless, usually less than 3 cm in diameter (3, 10), grow slowly, and are confined to the subcutaneous tissue (13, 18). Females are usually reported as being more commonly affected than males by a ratio of approximately 1.5:1 (10, 16); however, a recent report of 179 cases by Lan et al. (12) showed a very slight male predilection. Approximately 2% to 3% of tumors are multiple (10, 14), although multiple lesions have been reported in as many as 14% of patients in some series (16). Multiple lesions have been associated with Gardner syndrome, myotonic dystrophy, Curschmann-Steinert, Rubinstein-Taybi syndrome, and gliomatosis cerebri (19). Activating mutations in the β-catenin gene (a known oncogene for colon cancer), which inhibit degradation of the protein and lead to its nuclear accumulation, have been observed in about 80% of pilomatricomas and pilomatrix carcinomas (19). Rare, large, fungating lesions have also been reported (20).
Local excision is usually curative and recurrence is rare (10). Aggressive local behavior is only rarely
reported (17, 21). These aggressive lesions are termed pilomatrix carcinoma or calcifying epitheliocarcinoma of Malherbe (21). Clinically, they are more common in men (2:1) and occur in an older age group (21). Sassmannshausen and Chaffins reported a case of pilomatrix carcinoma in 2001 and reviewed the literature, identifying 72 previously reported cases: 26 demonstrated local recurrence and 8 demonstrated metastatic disease (22). Metastases have been noted to the lung, bone, and lymphatics, as well as disseminated (22). Bone invasion is exceedingly rare, with only a single reported case (10).
reported (17, 21). These aggressive lesions are termed pilomatrix carcinoma or calcifying epitheliocarcinoma of Malherbe (21). Clinically, they are more common in men (2:1) and occur in an older age group (21). Sassmannshausen and Chaffins reported a case of pilomatrix carcinoma in 2001 and reviewed the literature, identifying 72 previously reported cases: 26 demonstrated local recurrence and 8 demonstrated metastatic disease (22). Metastases have been noted to the lung, bone, and lymphatics, as well as disseminated (22). Bone invasion is exceedingly rare, with only a single reported case (10).
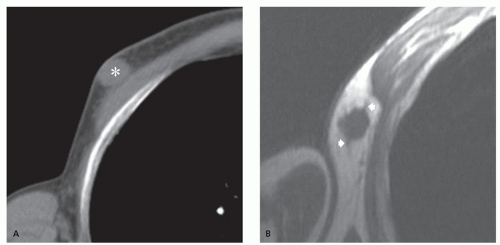
Microscopy shows differentiation toward hair matrix (10). The tumor consists of sheets and bands of epithelial cells in a connective tissue stroma (13). Shadow cells (which are histochemically similar to inner root sheath keratin) occur within nests of basophilic cells (13). A connective tissue capsule has been reported in more than 75% of patients (16). Lesions are subcutaneous, with a well-defined, sharp margin (13, 14). Calcification, which is more typically central, is seen in approximately 84% of lesions (11). The calcification is described as homogeneous speckles or sand-like. Ossification occurs in an estimated 20% of these and is more typically peripheral (11).
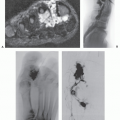
Radiographic calcification is less common than pathologic calcification, reported in 55% of cases, whereas at computed tomography (CT), identification of mineralization approaches that seen pathologically (Fig. 12.4) (16). At CT, images will demonstrate an attenuation similar to that of skeletal muscle, with mild to moderated enhancement (16).
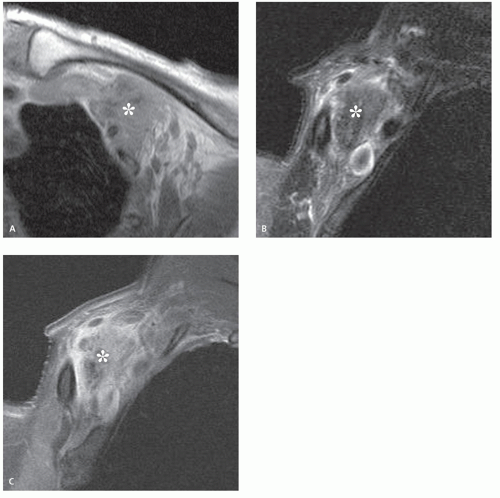
Limited MR imaging data notes the nonmineralized portions of the lesion to have an intermediate signal intensity on T1-weighted and fast spin-echo T2-weighted images, with variable patchy, rim, and internal reticular patterns of heterogeneous enhancement reported following contrast administration (Fig. 12.5) (14, 16, 18). Perilesional stranding was reported in three of five cases by Lim et al. (16).
Ultrasound will demonstrate a subcutaneous, heterogeneous, hypoechoic mass with internal echogenic foci in a scattered-dot pattern, a hypoechoic rim, and posterior shadowing (23). The posterior acoustic shadowing is seen in almost 90% of cases and is the result of the internal calcification (16). Lesions will also demonstrate internal hyperechoic echogenicity (88%), hypoechoic rim (65%), and perilesional hyperechogenicity (47%) (16). Lim et al. correlated the hypoechoic rim on ultrasound and the rim enhancement on MR imaging with the connective tissue capsule surrounding the lesion identified on pathological examination, and perilesional hyperechogenicity on ultrasound and stranding on MR imaging with perilesional inflammatory change (16).
Sweat Gland Tumors
Sweat glands are generally subcategorized into two types: eccrine glands, which produce abundant, clear, nonodorous sweat that reaches the skin through pores, and apocrine glands, which are always associated with hair follicles and produce small amounts of turbid, odorous milky sweat (24). Eccrine glands are distributed throughout the body and are especially abundant in the head, neck, and extremities, whereas apocrine glands are found primarily in the axilla, anogenital areas, mammary glands, and eyelids (25).
Although it is convenient to divide sweat gland tumors into those of eccrine or apocrine origin, their
histopathologic classification is more complex, with lesions frequently showing divergent differentiation (eccrine and apocrine) within a single neoplasm (26). For most sweat gland tumors, both benign and malignant counterparts are described. Some of these malignant sweat gland tumors may arise from preexisting, benign, adnexal neoplasms, whereas others arise de novo. They are named according to the benign tumors they resemble (26). Most of these tumors are rarely imaged. The more common sweat gland lesions that we have seen in our practices are described below.
histopathologic classification is more complex, with lesions frequently showing divergent differentiation (eccrine and apocrine) within a single neoplasm (26). For most sweat gland tumors, both benign and malignant counterparts are described. Some of these malignant sweat gland tumors may arise from preexisting, benign, adnexal neoplasms, whereas others arise de novo. They are named according to the benign tumors they resemble (26). Most of these tumors are rarely imaged. The more common sweat gland lesions that we have seen in our practices are described below.
Dermal Cylindroma
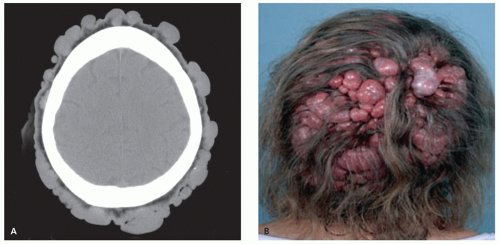
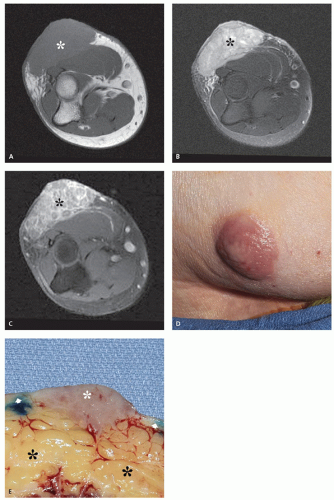
Dermal cylindroma is a slowly growing, benign, dermal tumor with immunohistochemical properties supporting eccrine differentiation (1, 27, 28). It is characterized by its clinical appearance and is only rarely imaged. Cylindromas are seen primarily on the head, neck, and scalp, with these locations accounting for about 90% of cases (28). Women are affected much more commonly than men (2-9:1) (1, 29). Lesions usually appear in early adulthood and appear as bulbous, violaceous masses, increasing in size and number with age (29).
KEY CONCEPTS
Dermal cylindroma is a slowly growing, benign, dermal tumor.
About 90% of lesions are found on the head, neck, and scalp.
Women are affected much more commonly than men (2-9:1).
Radiographs and CT will demonstrate a nonmineralized superficial mass.
Limited MR imaging of scalp lesions describes them as enhancing, pedunculated masses, generally isointense to brain on T1- and T2-weighted images.
Familial cases have been described and are typically associated with multiple lesions (28). Multiple lesions may be associated with autosomal dominant Brooke-Spiegler syndrome (familial cylindromatosis) (30).
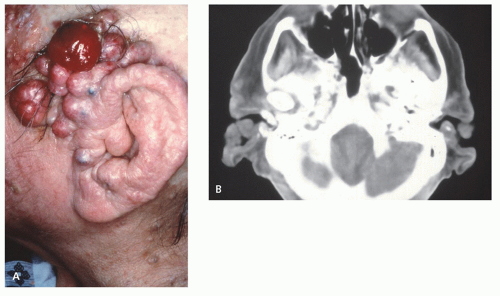
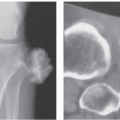
There is scant literature describing the appearance of this lesion. Radiographs and CT will demonstrate a nonmineralized, superficial mass (Figs. 12.6 and 12.7) (27, 29). Osseous calvarial defects have been reported (27, 30), as has intracranial extension mimicking a meningioma (30). Limited MR imaging of scalp lesions describe them as enhancing, pedunculated masses, generally isointense to brain on T1- and T2-weighted images, with focal heterogeneity in areas of necrosis and ulceration (29).
Spiradenoma
Spiradenoma is a nodular, benign, appendage tumor that recapitulates primitive eccrine dermal tubular and secretory epithelium (26). The term spiradenoma is derived from the Greek word for coil, to emphasize the prominent spiraling ducts identified microscopically (26). The lesion is closely related to cylindroma, with both lesions showing differentiation toward the secretory portion of the sweat gland (25).
Lesions are typically solitary and small (26), occurring in the face, trunk, or extremities, and found in the skin and subcutaneous tissue. They are most common in patients 20 to 40 years of age, with no gender predilection (31, 32). Multiple lesions may form linear or zosteriform configurations (26).
KEY CONCEPTS
Spiradenoma is a nodular, benign, appendage tumor that recapitulates primitive eccrine dermal tubular and secretory epithelium.
Lesions are typically solitary and small, occurring in the face, trunk, or extremities.
Spiradenoma is most common in patients 20 to 40 years of age, with no gender predilection.
Sonography will show an anechoic mass arising from the skin and subcutaneous fat.
MR imaging will demonstrate a subcutaneous cystic or solid nodule, with low signal intensity on T1-weighted images and intermediate-to-high signal intensity on T2-weighted and STIR images.
On ultrasound, the lesion has been reported to be an anechoic mass arising from the skin and subcutaneous fat, with posterior enhancement and peripheral Doppler vascularity (31, 33). The MR imaging of spiradenoma is limited to a few case reports and demonstrates a subcutaneous cystic or solid nodule, with low signal intensity on T1-weighted images and high, or occasionally intermediate-to-high, signal intensity on T2-weighted and short-tau inversion recovery (STIR) images (Fig. 12.8) (31, 34, 35). Han et al. (32) reported a patient with multiple lesions in a zosteriform distribution, showing extensive nodules in the cutis and subcutaneous tissue. Some of these lesions were scattered, while others were clustered, but separated from each other. They showed low signal intensity on T1-weighted images and high signal intensity on STIR mages (32).
Syringoma and Chondroid Syringoma
The term syringoma is used for a benign, adnexal lesion believed to originate from the ductal portion of the eccrine sweat gland (26). Lesions are generally small, measuring only a few millimeters in size (1 to 3 mm), and typically appear after puberty in the periorbital areas and the upper cheeks (25, 26, 36). Women are affected more commonly than men, and syringomas are more common in individuals of Asian descent (25). Lesions may be solitary, but are most frequently multiple, typically appearing as asymptomatic amber or flesh-colored papules (Fig. 12.9) (25, 26, 36). Syringomas usually appear in adolescence or early adulthood. On histologic examination, these small duct-like lesions are imbedded deep within the dermis, enveloped by fibrous tissue (36). Various treatments, including electrodessication, cryosurgery, chemical peeling, laser ablation, and surgery have been used (36). Because of their small size, these lesions are not imaged.
The chondroid syringoma is a related lesion, so named because it consists of a proliferation of ductal and tubular eccrine (or apocrine) epithelium with a characteristic chondroid stromal matrix (25). This lesion is also known as mixed tumor of the skin. Lesions are subcutaneous, solitary, slowly growing, and typically located in the head or neck (80%) (37, 38). In contrast to syringoma, chondroid syringoma is twice as common in men as in women and
is larger in size (37). Rare cases of malignant syringoma and chondroid syringoma are reported; however, reliable imaging criteria for distinguishing malignancy are not described.
is larger in size (37). Rare cases of malignant syringoma and chondroid syringoma are reported; however, reliable imaging criteria for distinguishing malignancy are not described.
There is scant literature addressing the imaging appearance of these tumors, because typical lesions are small and diagnosed clinically or without imaging. Lesions that are reported are those that are unusually large and/or in atypical locations. MR imaging will show a lesion localized to the subcutaneous tissue, with low-to-intermediate signal intensity on T1-weighted images and heterogeneous high signal intensity on fluid-sensitive images. The heterogeneous signal intensity has been attributed to areas of hemorrhage and/or fibrous tissue (39). Following contrast administration, heterogeneous enhancement is seen (37). On T1-weighted images, areas of increased signal intensity have been described, which correlate to globules of intralesional fat on histological examination (39). Histologic evaluation will also reveal a multilobulated structure, defined by fibrous septa, which separate a basophilic chondroid matrix and epithelial component, with foci of pinpoint hemorrhage (39). Foci of hemorrhage may also contribute to areas of increased T1-signal (Fig. 12.10). Ryu et al. (39) reported the sonographic features in a case involving the heel and noted the lesion to be hyperechoic, with a hypoechoic center and
linear hypoechogenic septa extending peripherally, with these septa showing prominent vascularity.
linear hypoechogenic septa extending peripherally, with these septa showing prominent vascularity.
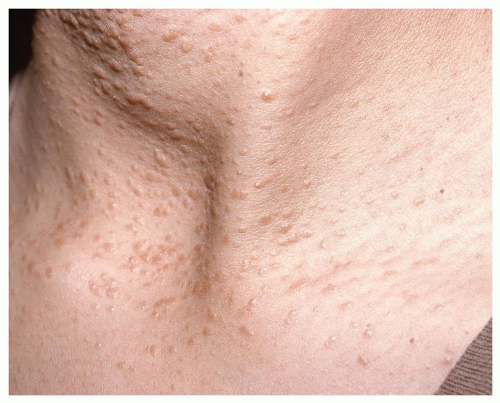 FIGURE 12.9 Syringoma: Clinical image of a young woman with multiple, small (1 to 3 mm) flesh-colored papules. |
KEY CONCEPTS
Syringoma is a benign dermal lesion believed to originate from the eccrine sweat gland.
Lesions are generally small, measuring only a few millimeters in size, and typically appear after puberty in the periorbital areas and in the upper cheeks of women.
Because of their small size, these lesions are not imaged.
Chondroid syringoma, also known as mixed tumor of the skin, consists of a proliferation of ductal and tubular eccrine (or apocrine) epithelium with a characteristic chondroid stromal matrix.
Chondroid syringomas are subcutaneous, solitary, slowly growing, and typically located in the head and neck (80%), and are twice as common in men.
MR imaging will show a lesion localized to the subcutaneous tissue, with low-to-intermediate signal intensity on T1-weighted images and heterogeneous high signal intensity on fluid-sensitive images.
On T1-weighted images, areas of increased signal intensity have been described, which correlate to globules of intralesional fat.
MALIGNANT SUPERFICIAL LESIONS
The soft tissue is relatively resistant to metastasis, and although it comprises about 40% to 50% of total body mass, soft tissue metastases are quite rare (1, 40). Any malignancy may disseminate to the skin, with 5% to 10% of all cancer patients developing skin metastases. Clinically numerous, small, hard, or rubbery nodules are present typically on the chest, abdomen, or scalp in adults over 40 years. Skin involvement is usually near the site of the primary tumor (1). Due to overall disease prevalence, breast is the most common primary cancer metastatic to skin in women (41). In men, melanoma, followed by lung cancer, is most common (41). Although cutaneous metastases are frequently identified in clinical practice, they are uncommonly imaged.
In this section we will review the common, superficial, metastatic lesions. We have included the appearance of intramuscular involvement where appropriate.
Metastatic Carcinoma
Solitary soft tissue metastasis presenting as a primary soft tissue tumor is rare. In a study of 1,421 patients presenting at two orthopedic oncology centers, Glockner et al. (42) identified only 25 (1.8%) such cases in patients with known primaries. The incidence of metastases in patients with no known primary was even lower, at 0.8%. Similar results were found by Abed et al. (43), reporting on a review of 7,935 patients presenting for evaluation of a soft tissue mass: only 100 (1.3%) presented with a soft tissue metastasis.
Soft tissue metastases may be seen in the subcutaneous tissues as well as in muscle. Although only limited reports are available, subcutaneous metastases in patients with carcinoma are less common than muscle metastases by a factor of 1.8 to 3.5:1 (43, 44). Muscle metastases may present in areas of previously documented trauma, suggesting that muscle injury may alter muscle physiology and increase susceptibility to the development of soft tissue metastases (40).
Adenocarcinoma is the most common soft tissue metastasis, followed by squamous cell carcinoma and metastatic melanoma (44, 45). Patients with soft tissue metastases typically have clinical features that will overlap with those of patients with primary soft tissue tumors (44). Although soft tissue metastases may occur
anywhere, they are most frequent to the abdominal wall, back, thigh, chest wall, and shoulder (45).
anywhere, they are most frequent to the abdominal wall, back, thigh, chest wall, and shoulder (45).
KEY CONCEPTS
Solitary soft tissue metastases are rare, representing about 1% of patients presenting to orthopedic oncology centers.
Subcutaneous metastases are less common than muscle metastases by a factor of 1.8 to 3.5:1.
Adenocarcinoma is the most common soft tissue metastasis, followed by squamous carcinoma and metastatic melanoma.
MR imaging will show an enhancing, well-defined, round to ovoid mass, with marked enhancement.
Lesions are usually isointense to skeletal muscle on T1-weighted images and hyperintense to normal, unaffected muscle on fluid-sensitive sequences.
Associated surrounding perilesional edema-like signal has been reported in 2% to 78% of patients.
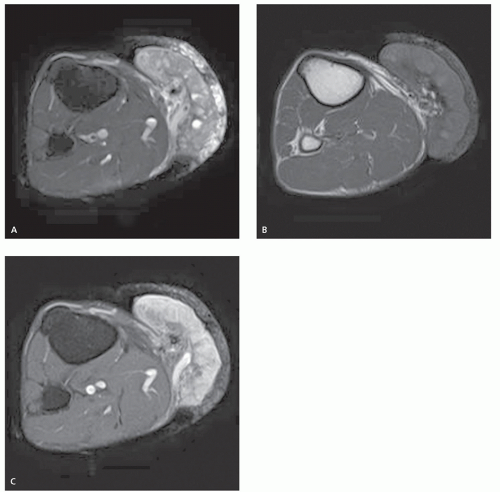
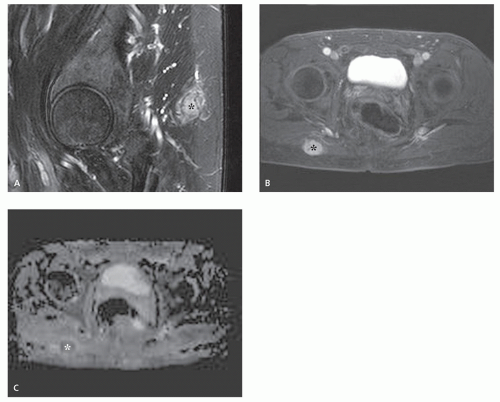
There is limited literature detailing the imaging of metastatic disease, typically highlighting intramuscular disease (40, 46, 47, 48). It has been our experience that while superficial (subcutaneous) lesions are less common, their imaging appearance will mirror that of intramuscular disease. Intramuscular metastases are typically well-defined, round to ovoid masses, with marked enhancement (40, 46). Lesions are typically isointense to skeletal muscle on T1-weighted images and hyperintense to normal unaffected muscle on fluid-sensitive sequences (46, 47, 48).
The presence of associated surrounding perilesional edema-like signal has been reported with varying frequency in 2% to 78% of patients; however, we have found this to be a common finding (46, 47). Necrosis has been reported in 67% of patients (47), being more frequent in larger lesions (Figs. 12.11 and 12.12). Apparent diffusion coefficient (ADC) maps will typically show low-to-moderate ADC values reflecting increased cellularity (Fig. 12.13) (46).
Merkel Cell Carcinoma (Neuroendocrine Carcinoma of the Skin)
Merkel cell carcinoma is a rare, highly malignant, cutaneous tumor of the skin (49) characterized by aggressive behavior, rapid growth, and early lymphatic and hematogenous metastases (50). Merkel cell carcinoma was first described by Toker (51) in 1972 as trabecular carcinoma of the skin, in a report of five patients with a distinctive cutaneous neoplasm. Toker noted that although these patients were initially diagnosed as having metastatic carcinoma, some with repeated local recurrences, none had an identified primary lesion. Merkel cell carcinoma is now best classified on the basis of its immunocytologic characteristics as neuroendocrine carcinoma of the skin (49), and is so classified in the 2006 World Health Organization (WHO) Classification of Skin Tumors (52). On the basis of immunohistochemical and ultrastructural studies, the tumor is thought to likely originate from the Merkel cell, although a direct histogenetic link is unproven (49, 52, 53). Other names for this lesion include primary small-cell carcinoma of the
skin and cutaneous APUDoma (amine precursor uptake and decarboxylase) (52).
skin and cutaneous APUDoma (amine precursor uptake and decarboxylase) (52).
Merkel cell carcinoma is a rare lesion with a WHO-estimated incidence of less than 500 new cases per year in the United States (52). It occurs two to three times more frequently in men than in women, with typical patients presenting in the seventh decade of life (49, 50, 52, 54). It is most common in Caucasians and is rare in blacks (49, 52). The lesion occurs most often in sun-exposed skin and is usually seen in the neck (50%) and extremities (40%) (50, 52, 55). There is an increased incidence in immunocompromised individuals. In addition, there is an increased prevalence of second neoplasms occurring in patients with Merkel cell, before, concurrent with, or following diagnosis (49). Ultraviolet exposure has also been found to be directly proportional to the risk of Merkel cell carcinoma. Patients with psoriasis, who have been previously treated with UVA radiation and methoxsalen, were found to have a 100-fold increased incidence, as compared to the general population (56). Although the lesion is rare, its incidence rates have increased threefold over the 1986 to 2001 period (54).
Clinically, patients will present with a nontender, cutaneous, red-to-purple, subcutaneous nodule (50, 55). Up to 30% of patients will have regional nodal spread at time of
initial presentation and 1% to 4% will have hematogenous metastases (57, 58). The lesion arises in the dermis and extends into the subcutaneous fat (50). The overlying epidermis is commonly intact (50). Distant metastases are most frequent to lymph nodes, liver, bone, brain, lung, and skin in decreasing order (55). Overall local recurrence rate is approximately 25% to 45%, with regional lymph node metastases occurring in approximately 67% of patients during the course of their disease (57, 59). Distant metastases will develop in half of patients within 2 years (59).
initial presentation and 1% to 4% will have hematogenous metastases (57, 58). The lesion arises in the dermis and extends into the subcutaneous fat (50). The overlying epidermis is commonly intact (50). Distant metastases are most frequent to lymph nodes, liver, bone, brain, lung, and skin in decreasing order (55). Overall local recurrence rate is approximately 25% to 45%, with regional lymph node metastases occurring in approximately 67% of patients during the course of their disease (57, 59). Distant metastases will develop in half of patients within 2 years (59).
KEY CONCEPTS
Merkel cell carcinoma is a rare, highly malignant, cutaneous tumor of the skin.
Also termed neuroendocrine carcinoma of the skin, primary small-cell carcinoma of the skin, and cutaneous APUDoma.
Most frequent in older Caucasian men, typically in sunexposed skin; neck (50%) and extremities (40%).
Arises in the skin and extends into the subcutaneous fat.
Local recurrence in 25% to 45%: regional lymph node metastases in 67% of patients; distant metastases will develop in half of patients within 2 years.
Imaging will demonstrate a focal mass arising from the skin and extending into the subcutaneous fat.
The adjacent skin will be thickened and inflamed and reticulation may be seen in the adjacent subcutaneous fat, representing lymphatic spread.
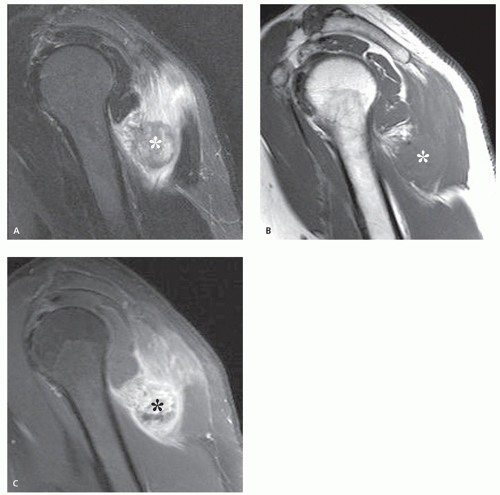
MR imaging will demonstrate a focal mass arising from the skin and extending into the subcutaneous fat. Individual lesions vary greatly in size: often small, but large lesions of 7 to 10 cm are not uncommon. Lesions will show a nonspecific signal intensity, being isointense or slightly hyperintense to muscle on T1-weighted images and hyperintense on T2-weighted and STIR images (Fig. 12.14) (50). Diffuse enhancement will be seen
following contrast administration (50). Larger lesions tend to be more heterogeneous and may demonstrate central necrosis and hemorrhage (57). The adjacent skin will be thickened and inflamed; reticulation may be seen in the adjacent subcutaneous fat (60). Reticulated stranding associated with these lesions was correlated by Anderson et al. (50) and found to represent lymphangitis carcinomatosa and soft tissue lymphatic metastases. Additional masses may be present, as may enlarged, associated lymph nodes, with regional nodal metastasis commonly identified in patients being evaluated for tumor staging (50).
following contrast administration (50). Larger lesions tend to be more heterogeneous and may demonstrate central necrosis and hemorrhage (57). The adjacent skin will be thickened and inflamed; reticulation may be seen in the adjacent subcutaneous fat (60). Reticulated stranding associated with these lesions was correlated by Anderson et al. (50) and found to represent lymphangitis carcinomatosa and soft tissue lymphatic metastases. Additional masses may be present, as may enlarged, associated lymph nodes, with regional nodal metastasis commonly identified in patients being evaluated for tumor staging (50).
On CT imaging, lesions are reported to have a tissue attenuation similar to or slightly greater than that of skeletal muscle (55), Associated stranding is reported in 25% of cases (Fig. 12.15) (55). Ultrasound will show a hypoechoic nodule (or nodules) arising from the skin and extending into the subcutaneous fat, as seen on other imaging modalities. Varying degrees of posterior acoustic shadowing are seen (61).
Local recurrence, as well as regional and distant metastasis, may be identified with somatostatin receptor scintigraphy (49). Octreotide, a somatostatin analogue tagged with indium-111, can be used to identify clinically occult lesions (49). Positron emission tomography with computed tomography (PET/CT) has also proved to be useful in detecting and staging this lesion.
Malignant Melanoma
Malignant melanoma is not an uncommon tumor, with over 50,000 new cases each year (62). In its early stages,
melanoma is curable by surgical excision, which accounts for about 85% of cases; the remaining patients presenting with locally advanced or metastatic disease (63). Metastases are most common to the lung, liver, bone, brain, and subcutaneous tissue (64). In a review of 53 patients with melanoma, Patten et al. (64) reported that 38% of patients had evidence of subcutaneous metastatic disease by CT, and subcutaneous metastases were the only evidence of widespread disease in 11% of patients. This pattern of metastatic spread is especially true for patients with Clark level IV or V disease (depth on tumor invasion into the deep dermis, or through the dermis into the subcutaneous fat), representing relatively deep disease (64).
melanoma is curable by surgical excision, which accounts for about 85% of cases; the remaining patients presenting with locally advanced or metastatic disease (63). Metastases are most common to the lung, liver, bone, brain, and subcutaneous tissue (64). In a review of 53 patients with melanoma, Patten et al. (64) reported that 38% of patients had evidence of subcutaneous metastatic disease by CT, and subcutaneous metastases were the only evidence of widespread disease in 11% of patients. This pattern of metastatic spread is especially true for patients with Clark level IV or V disease (depth on tumor invasion into the deep dermis, or through the dermis into the subcutaneous fat), representing relatively deep disease (64).
KEY CONCEPTS
Metastases are seen in 15% of patients with melanoma.
In patients with metastases, almost 40% will have involvement of the subcutaneous tissue.
Subcutaneous lesions will be the only evidence of widespread disease in 11% of patients.
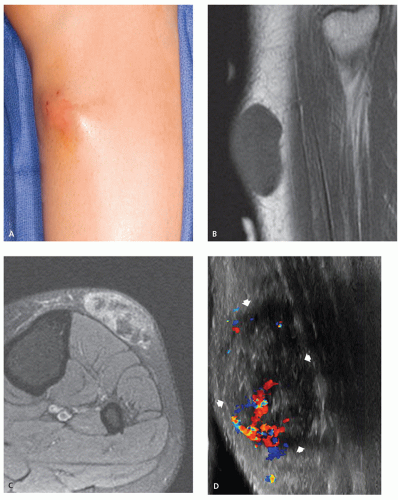
On sonography, superficial metastatic melanoma typically will present as a well-defined, hypoechoic nodule with mild-to-moderate heterogeneity and enhanced acoustic through transmission.
Doppler sonography will show internal arterial color flow in most, but not all, lesions.
MR imaging may show increased signal intensity on T1-weighted images.
Ocular melanoma accounts for about 5% of all melanoma (65, 66). Metastases are much more common in this group. In a report of 110 patients with metastatic ocular melanoma, Lorigan et al. (67) reported skin and subcutaneous metastases in 17% of patients, with only 2% of patients having metastases limited to the skin or subcutaneous region. In contrast, liver metastases were noted in 92% of patients.
On sonography, superficial metastatic melanoma typically will present as a well-defined, hypoechoic nodule with a smooth or lobulated contour, mild-to-moderate heterogeneity, and enhanced acoustic through transmission (68). Doppler sonography will show internal arterial color flow in most (72%), but not all, superficial metastatic lesions (Fig. 12.16) (68).
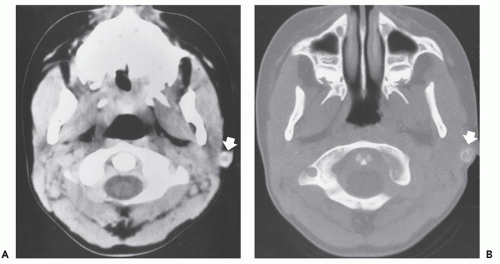
On CT scans, lesions are easily identified within the subcutaneous adipose tissue (Fig. 12.17). Intramuscular lesions can be more difficult to identify and contrast-enhanced imaging will generally allow better definition of tumor margins, with lesions often showing increased attenuation relative to normal muscle. On MR imaging, lesions with a higher melanin content will show T1-shortening due the paramagnetic properties of melanin, and demonstrate increased signal intensity on T1-weighted images (60, 69, 70). This increased signal intensity is best appreciated on fat-suppressed T1-weighted images. Melanoma lesions will also show T2-shortening; however, this effect is less pronounced and lesions typically demonstrate an intermediate-to-increased signal intensity on T2-weighted and fat-suppressed, fluid-sensitive sequences (Fig 12.18) (60, 70, 71, 72, 73).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree