Cancer patients experience pain at significant rates but are often undertreated—it is estimated that less than 1% of eligible cancer pain patients receive appropriate targeted drug delivery to address their pain. Cancer pain is often managed with systemic opioid treatment; however, this approach is limited in treating pain adequately and carries significant side effect risk profiles. Successful treatment of pain is closely tied to better oncologic outcomes as well as better measures on assessments of quality of life for cancer patients. Placement of intrathecal pain pumps represent a safe and effective way to manage pain in cancer patients. We describe the process of placing intrathecal pain pumps in an interventional radiology suite. This method of pump placement represents a minimally invasive approach to long term and continuous pain relief. Intrathecal pain pumps help maximize pain control for patients experiencing refractory pain due to disease process or treatments associated with malignancy.
Introduction
In patients with active cancer-related pain, there is strong evidence that intrathecal therapy is more effective than opioid therapy delivered systemically. Intrathecal pain pumps are also indicated for patients who are experiencing residual pain from malignancy that is currently in remission, or pain associated with treatment for cancer like chemotherapy or radiation. In particular, patients who have chronic pain and are experiencing inadequate pain relief with systemic opioids or who suffer from adverse side effects are good candidates for pump placement. Intrathecal pain pumps should be considered first line for refractory pain in patients where evidence-based biomedical therapies have failed, and the patient needs continued pain relief.
Other considerations include the location of the patient’s pain, the type of pain they are experiencing, and patient life expectancy. Patients with dermatomal pain benefit from targeted intrathecal placement. Both neuropathic and nociceptive pain can be addressed by intrathecal pain pumps using different medications, which will be discussed in detail below. Patient life expectancy, taken into consideration along with use of pain management for palliative effect or the presence of residual chronic pain was noted by Deer et al. to define categories that can be used to help guide the decision-making process for selecting patients who are good candidates for pain pumps.
Absolute contraindications to the procedure include infection, allergy to the medication, obstructed CSF flow, or active substance abuse. Lab work showing a white blood cell count below 2 × 10 9 /L, an absolute neutrophil count below 1000 cells/mm 3 , or platelets below 50,000 × 10 9 /L are all contraindications to continuing with the procedure.
Relative contraindications include current anticoagulation use, or certain disease comorbidities. For patients on anticoagulation, moving forward with the procedure is possible as long as the patient has stopped anticoagulation prior to the procedure according to the half-life of the specific drug. They may restart their medication 24 hours after the catheter is placed. Other disease comorbidities to consider include any conditions that affect respiratory drive, including obstructive sleep apnea, cardiac disease, pulmonary disease, or advanced age. However, for patients who may already be opiate tolerant using oral medications, these conditions do not represent an absolute contraindication given that there is not a significant change in respiratory status from oral to intrathecal opioid delivery. Other considerations include kidney function if planning to use ziconotidine, as there is an expected increase in creatinine kinase that must be monitored regularly. Other medication interactions to carefully review include drugs that affect the central nervous system like patients on benzodiazepines, antidepressants, anticonvulsants, or muscle relaxants. Physician discretion on the dosage of these medications and their interactions with opioids must be carefully considered.
Clinical evaluation of the patient
Past medical history
Once a patient with significant pain associated with a known malignancy or related to treatment for malignancy is identified, the first clinic visit is an opportunity to get to know the patient and understand their experience of pain.
A thorough medical history is important to better understand the type of malignancy the patient has, as well as any history of chemotherapy or radiation treatments to treat the malignancy. In addition to pain due to direct invasion of nearby structures, patients may experience pain secondary to chemotherapy induced neuropathy or radiation treatments. It is also important to note if the patient is in remission, and if so, the likelihood of recurrence. This can help determine if the intrathecal pump will need to be refilled over time, or if the placement is short term and intended for palliative pain relief. Other aspects of medical history to note include any medical conditions that may represent contraindications to opiate use.
Surgical history of note would include any prior spinal procedures. Psychiatric history is also important to collect, as any history of psychosis represents a contraindication to using ziconotide.
Medication history should include a careful accounting of any previous or current opioid use, as well as the duration and dosages of all opioids. Patients may require a titration process if they are on current opioid pain treatment, or if they are not opiate naïve. Further medications that may warrant concern include any drugs that affect the central nervous system, as this may cause side effects when combined with opioid treatment intrathecally.
Social history should note any recreational drug use history and alcohol use history, as chronic use may also affect opiate effectiveness. It is also worth noting what psychosocial support factors the patient has, as this can help determine if the patient is a good candidate for the procedure. For example, the patient will need reliable transport for follow-up appointments or pain pump refills.
Physical exam
The exam should focus on elucidating the location and type of pain the patient is experiencing. Is the pain local, diffuse, or global? This can help direct the level at which the intrathecal catheter is placed in the spinal column. The exam should also include careful examination of the spine and location for initial incision as well as intrathecal catheter placement. Any scoliosis or lordosis can make needle placement difficult, and spinal abnormalities may inhibit CSF flow, so noting any spinal anatomical variation is important. The abdomen and hips should be carefully examined to determine a location for reservoir pump placement. Ideally, patients have adequate body habitus to accommodate the bulk and weight of the reservoir placed in the abdomen. However, in patients who are cachectic, the reservoir can also be placed in the lower back or flank to ensure there is adequate tissue to support the device after placement.
Labs
Important screening labs prior to the procedure include a complete blood count to rule out any current infection or severe anemia. Coagulation studies are also important, especially if the patient is on blood thinners and will need to stop these drugs prior to the procedure. A complete metabolic panel is also important to ensure the patient can adequately clear any medications used in the pain pump. Finally, patients should be screened for diabetes.
Imaging
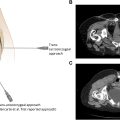
Relevant imaging for preprocedure planning focuses on assessing the spinal anatomy to help plan the placement approach. MRI of the thoracic and lumbar spine is the most valuable modality, but CT can be considered if MR is unavailable.
Preparing for the procedure
Equipment needed
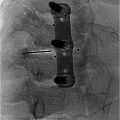
There are 4 main types of pumps on the market. These include a percutaneous catheter attached to an external pump, which may be tunneled beneath the skin from the access site or not tunneled. These pumps are better for patients with limited mobility or a short life expectancy as they are easy to access. A second type of pump is an implanted catheter with a subcutaneous injection port connected to an external pump. The third is a fully implanted and fixed rate system. Finally, there is a fully implanted and programmable system. Selection between these devices is determined in part by patient life expectancy, ease of refilling the site long term, and patient tolerability of the procedure.
Other equipment needed includes sutures for attaching the catheter anchor, securing the pump in placing, closing the fascia and subcutaneous skin. We recommend using Vancomycin powder in the pocket, as well as using a TYRX patch, which is placed into the pocket on top of the Vancomycin powder before the pump is put into place. Patients should also be provided an abdominal binder postoperatively.
Prior to the procedure
Patients should be given antibiotic prophylaxis, as this reduces the risk of infection associated with the procedure. While the patient is in the preoperative area, mark the site of implantation while they are seated. Marking the patient while they are reclined may cause incorrect positioning once the patient is seated or standing.
Procedural steps
The patient is placed in a lateral decubitus position with slight spinal flexion. The site where the pump was marked to be placed should be visible.
The patient can be moderately sedated or awake with extensive local anesthetic used so they can provide real time feedback of spinal cord sensation. However, for pediatric patients or patients with significant tremor or spasticity, general anesthesia is preferred. We prefer general anesthesia in most cases due to the painful nature of the procedure.
Prepare the skin using povidone-iodine or chlorhexidine-containing products. The doctor should double glove.
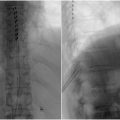
Initial needle entry is to inject local anesthetic into the skin and subcutaneous tissue by forming a wheal and then tracking the needle along the planned site of entry. The entry should be paramedian and 1 vertebral body level below the planned lumbar entry point, which should be below the level of the conus medullaris. Confirm location of the planned entry and catheter placement under fluoroscopy. An AP view can be used to help plan the trajectory from the skin entry to point to the interlaminar space. Once sufficiently anesthetized, a 16g Tuohy introducer is advanced through the fascia, angled 10°-15° laterally toward the midline, and is advanced cephalad. Continue advancing and adjusting Tuohy trajectory until the epidural space is reached, which will be noted by the loss of resistance. Once the epidural space is entered, rotate the bevel of the needle 90° toward midline until it is parallel to the dural fibers, which will help reduce the risk of postdural puncture headache. Advance the needle into the intrathecal space under lateral imaging to visualize the depth, and confirm once in position by removing the inner stylet to see CSF flow.
Once the introducer has been confirmed in place under fluoroscopy, insert the catheter and guidewire through the needle. Tilt the needle caudally, and advance the catheter caudally under fluoroscopy until it is at the desired spinal level. Figure 1 Placing the catheter tip close to the target receptors of spinal segments associated with the dermatome, sclerotome, or viscerotome of the primary area of pain provides greatest pain relief. Placement is often between T10 to T12, but can be adjust according to patient’s report of pain. Confirm CSF continues to flow by aspirating 0.5-1mL of CSF with a needle and syringe from the catheter access lumen.