The Altered Breast: Pregnancy, Lactation, Abscess Post-Biopsy Changes, Mastectomy, Radiation, and Implants
Breast Imaging During Pregnancy and Lactation
The breast undergoes significant changes during pregnancy and lactation. In the first few weeks of a pregnancy, there is a proliferation of ducts and lobules and an increase in secretions, as well as vascular engorgement and breast enlargement. As the pregnancy progresses, the lobular acini enlarge and become filled with colostrum. Several days after delivery and the cessation of placental and luteal hormone production, the pituitary gland begins to secrete prolactin, which causes the lobular acinar cells of the breast to begin to produce true milk. Under the stimulation of nursing, a neurologic loop causes the secretion of prolactin to continue. The neurologic pathway also causes the release of oxytocin from the pituitary, which in turn stimulates the hypertrophied breast myoepithelial cells, whose contraction helps to propel the milk down the ducts.
Mammography During Pregnancy
In general we try to avoid mammography during pregnancy. This is not completely contraindicated, but there should be a “compelling” reason for x-ray imaging of the breast during pregnancy. Fortunately, the fetus receives little or no radiation exposure if mammography is needed.
Fetal Radiation Exposure During Mammography Is Inconsequential
Concerns have been raised over possible scatter radiation to the fetus from a mammogram. Fortunately, using dedicated mammography equipment, calculations of radiation exposure to the uterus suggest that virtually no radiation would reach the fetus. This was determined in the following manner. In the craniocaudal (CC) projection, the primary beam that passes through the film and screen is attenuated by the cassette holder and plate on the other side of the breast. These permit approximately 0.002% of the incident radiation to pass through (approximately 0.2 mR). Assuming the fetus is 10 cm deep within the body under the film holder and plate, and because these tissues attenuate the radiation by a factor of 2 (or more) per centimeter, the dose to the uterus would be about 0.001% of the entry dose in the CC projection. Thus, a mammogram performed
in the CC projection would result in 0.0002 mR to the uterus.
in the CC projection would result in 0.0002 mR to the uterus.
Scatter radiation would add to this dose. Scatter has been measured at a distance of 12 inches from the breast surface and amounts to 1 mR per film. Assuming that there is no tissue attenuation and that the distance to the uterus is 8 inches, the dose from scatter would be approximately 2.25 mR. The attenuation of 20 cm of tissue is 1 million (i.e., 20 half-value layers). Therefore, the scatter reaching the uterus amounts to about 0.000002 mR.
Because the primary beam is directed away from the uterus in the lateral projection, scatter would be the primary source of exposure to the uterus. Thus, the dose to the uterus for each breast, from a two-view study, amounts to approximately 0.0002 mR (0.0004 mR for both breasts). Natural background radiation exposes the fetus to approximately 2 mR each week. Thus, the potential radiation exposure of a fetus from a mammogram is 5,000 times less than the weekly exposure from normal background radiation (essentially zero) (Webster E, personal communication, 1995).
Although virtually no radiation (scatter or direct) can reach the fetus from a mammogram, were the child to be born with genetic or hematologic problems, the question of the role of the mammogram would likely be raised, regardless of the scientific evidence. Nevertheless, if the mammogram might provide important information for patient care, then we will obtain first mediolateral oblique (MLO) views (to try to limit the exposure) and then add CC views should a significant question be raise by the MLOs.
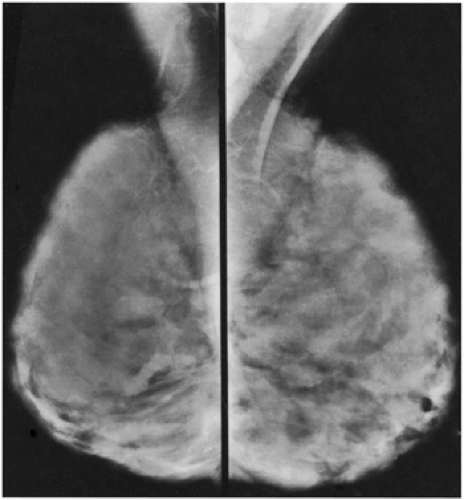
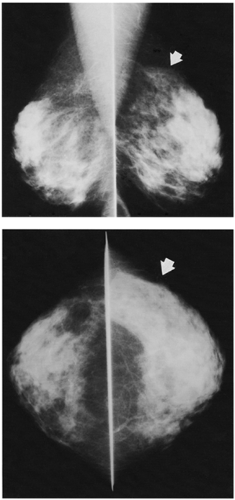
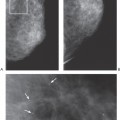
Unlike the findings during lactation, we have not found that the breast is particularly denser at this time (Fig. 18-1), but no rigorous scientific study has been done to evaluate this.
We do not feel that screening is indicated during this period. Most pregnant women are below the age for routine screening. Women at high risk should be addressed on a case-by-case basis. If a woman has a solitary duct discharge with either clear or bloody fluid during pregnancy, we would consider doing a mammogram to look for calcifications that might be associated with ductal carcinoma in situ (DCIS). There are few other indications that would prompt us to do a mammogram in a pregnant woman.
Usually when patients are referred for breast imaging during pregnancy it is for a solitary mass, and this can be evaluated using ultrasound (Fig. 18-2). An ultrasound-guided core biopsy can usually be accomplished if the lesion proves to be solid. Unless a cancer is discovered and it is important to try to assess its extent, mammography is not indicated. The simplest way to gauge the need for a mammogram during pregnancy is to remember that the mammogram is truly a screening test to look for unsuspected disease (cancer); it has little value as a diagnostic study. Recognizing this important point makes it easier to determine when to use mammography appropriately.
Radiation to the Breast From Mammography During Lactation
Although there are no direct data that contraindicate the use of mammography during lactation with regard to breast irradiation, concerns have been raised over the possible carcinogenic effects of radiation on the more active, proliferating breast epithelium. We generally avoid routine screening during pregnancy and lactation. If a mammogram is indicated
during this period, however, the theoretical risk, which is not quantifiable if it exists at all, should be balanced against the potential benefit. It can be argued that mammography is not of great benefit at this time because it is primarily a screening technique; if a patient has a significant clinical abnormality, it would have to be resolved regardless of the results of the mammogram. The presence of calcifications, however, might prompt earlier intervention.
during this period, however, the theoretical risk, which is not quantifiable if it exists at all, should be balanced against the potential benefit. It can be argued that mammography is not of great benefit at this time because it is primarily a screening technique; if a patient has a significant clinical abnormality, it would have to be resolved regardless of the results of the mammogram. The presence of calcifications, however, might prompt earlier intervention.
Mammography During Lactation
During pregnancy the lobules multiply and enlarge in preparation for lactation. As the breast prepares for lactation, fat droplets begin to accumulate in the epithelial cytoplasm. The intralobular stromal elements diminish and are crowded out and replaced by the enlarging lobules, which expand to touch one another. With the onset of lactation, secretions into the acini become profuse. The multiplication and enlargement of the lobules increase the x-ray attenuation of the breast tissues. Swinford et al found few changes on the mammogram during pregnancy relative to mammograms prior to pregnancy (1), but they drew their conclusions based on the appearance of the breasts among different women during pregnancy but before lactation (six women), during lactation (seven women), and in five women who were 1 week to 5 months following the cessation of lactation. Based on their work (with admittedly small numbers), it appears that the radiographic density of the breast is not particularly increased during pregnancy. All of the women who were lactating had dense breasts, but four of these women had dense breast tissue prior to their pregnancy. Among the six lactating women who had prior mammograms, four had no change from their prepregnancy mammograms, while two showed increased density. The five women who had ceased lactating appeared to have returned to their prepregnancy pattern.
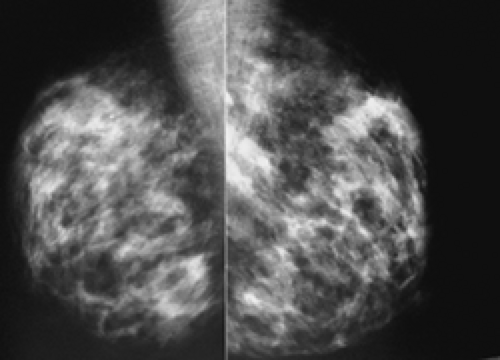
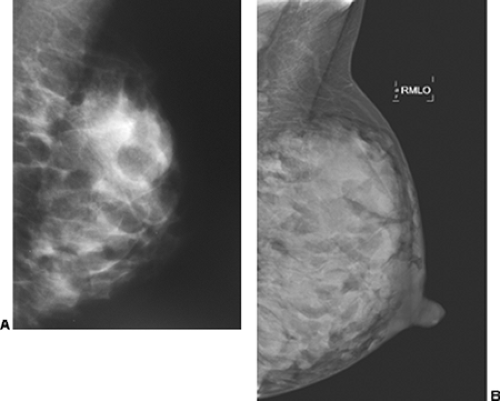
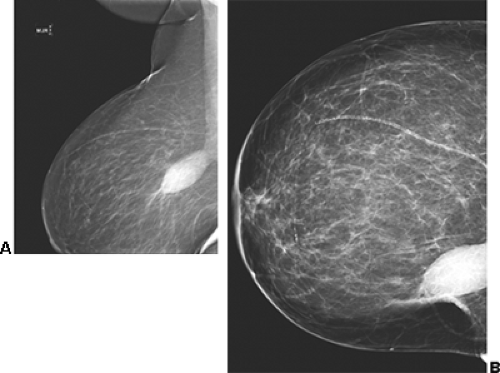
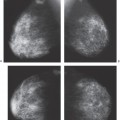
In our experience, the mammographic density of the breast can increase dramatically with lactation. This is most apparent in the breast that was predominantly fat before lactation (Fig. 18-3). Lobular enlargement produces numerous lobulated and coalescent densities on the mammogram (Fig. 18-4). Although never specifically studied, this increase in heterogeneous densities likely reduces the sensitivity of mammography for detecting breast cancer.
The lactating breast produces special problems for breast imaging due to the hypertrophic changes that are present. Routine screening should probably be avoided while a woman is lactating. The sensitivity of the mammogram for
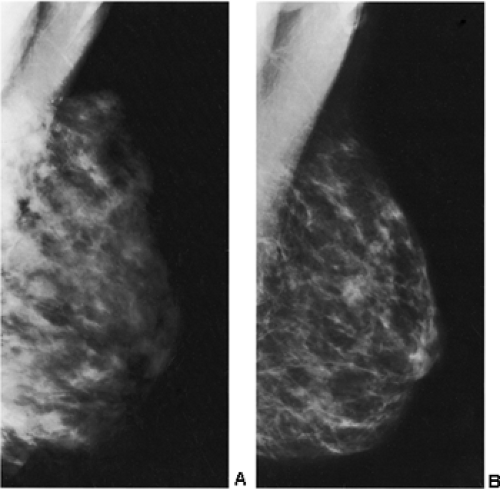
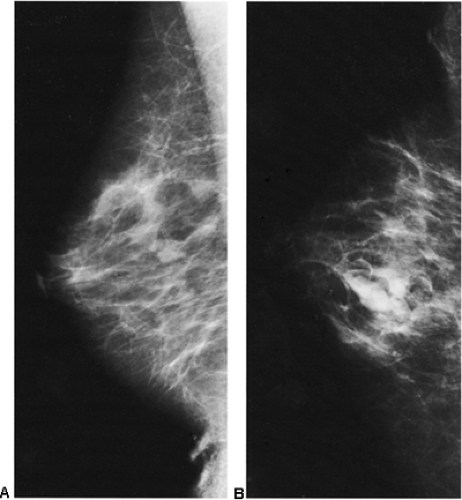
detecting cancer earlier is almost certainly reduced, and some observers have suggested that the breast may be more sensitive to radiation. If diagnostic imaging is needed to evaluate a palpable abnormality, ultrasound is used to determine whether the abnormality is cystic or solid. If a mammogram is indicated, it is preferable for the patient to nurse immediately before the mammogram to decompress the breast and permit better compression. Although we try to have the patient nurse just prior to the mammogram, on occasion this has not been possible, and we have found it is not unusual to see obviously enlarged, dense lobules full of milk (Fig. 18-5).
detecting cancer earlier is almost certainly reduced, and some observers have suggested that the breast may be more sensitive to radiation. If diagnostic imaging is needed to evaluate a palpable abnormality, ultrasound is used to determine whether the abnormality is cystic or solid. If a mammogram is indicated, it is preferable for the patient to nurse immediately before the mammogram to decompress the breast and permit better compression. Although we try to have the patient nurse just prior to the mammogram, on occasion this has not been possible, and we have found it is not unusual to see obviously enlarged, dense lobules full of milk (Fig. 18-5).
In a case report Stucker et al (2) reported on a case of a woman who was found to have diffuse intraductal calcifications by mammography 5 weeks after the cessation of lactation; at biopsy they were due to “lactational change.”
If there is no compelling reason to do a screening study sooner, we recommend waiting 3 months following the cessation of lactation before resuming screening, but this is based on anecdotal experience and not science.
Value of Breast Imaging in the Pregnant or Lactating Patient
Mammography can demonstrate abnormalities in pregnant women. What has never been shown is how the results of the mammogram influence the management of the patient. Liberman et al reported on 21 women (ages 24 to 41) who had mammograms during pregnancy (3). All of the women had clinically evident abnormalities. In 21 (91%) there was a palpable lump; 1 patient had a discharge; and 1 had diffuse enlargement of one breast with skin thickening. One woman proved to have bilateral cancers, and one had two synchronous lesions in the same breast. The mammogram showed an abnormality in 18 (78%) of the women.
In another study Ahn et al (4) found that a mammogram demonstrated an abnormality in 13 of 15 (86.7%) patients with breast cancer despite the fact that they had dense breasts. The mammograms revealed the following findings:
Five patients with a mass alone
Two patients with masses with calcifications
Two patients with calcifications and axillary adenopathy
One patient with a mass with axillary adenopathy
One patient with calcifications alone
One patient with an asymmetric density
One patient with diffuse skin and trabecular thickening
Ultrasound in the Management of the Symptomatic Pregnant or Lactating Patient
In the study by Ahn et al (4), ultrasound revealed masses in all 19 of the 19 women scanned. The sonograms showed the following:
15 cancers had irregular shapes.
16 cancers had irregular margins.
11 cancers had a “parallel orientation.”
14 cancers had complex internal echo patterns, including “marked cystic [anechoic] components” in 4.
12 cancers demonstrated posterior acoustic enhancement.
They concluded that “although a mass could not be discernible by mammography because of increased radiodensity during pregnancy or lactation, calcification, asymmetric density, axillary lymphadenopathy, and skin and trabecular thickening were helpful for diagnosis of pregnancy-associated breast cancer. The sonographic findings of a solid mass with posterior acoustic enhancement and a marked cystic component were somewhat different from the appearance of breast cancer in nonpregnant women, possibly because of the physiologic changes of pregnancy and lactation.”
Because there is no evidence of any detrimental effect from ultrasound, its use during pregnancy and lactation is attractive. As with any breast problem, ultrasound may be of value in differentiating cystic from solid masses. There are no good prospective data demonstrating that it can differentiate benign solid masses from malignant solid masses, and it should not be relied on for this purpose. The unanswered question is whether any breast imaging evaluation actually influences the care of these patients. Certainly, as in the nonpregnant individual, x-ray imaging and ultrasound can be used to guide needle procedures.
Effect of Pregnancy on Breast Cancer
The data suggest that an early first full-term pregnancy reduces a woman’s risk for the subsequent development of
breast cancer. A woman who has her first child by age 18 has one-third to one-half the risk of developing breast cancer as a woman who had her first full-term pregnancy after age 30. Nulliparous women are also at increased risk. The data suggest that there are stem cells that are involved in the development and differentiation of the immature lobule. Since these cells are “pleuripotential,” they are likely more susceptible to malignant transformation from exposure to carcinogens. Once the lobules have differentiated there are likely fewer stem cells, and the risk from exposure to carcinogens likely diminishes. A full-term pregnancy causes lobular differentiation in preparation for lactation. Consequently, a full-term pregnancy at an early age, resulting in lobular differentiation, may reduce the window of opportunity during which a carcinogen could have an effect on the epithelium. Differentiation takes place over a longer period of time if a pregnancy does not hasten the process. Although an early full-term pregnancy does not eliminate the risk of breast cancer, the rapid completion of lobular development likely narrows the window of opportunity during which the proliferating cells of the terminal duct are more likely to be susceptible to damage from a carcinogen.
breast cancer. A woman who has her first child by age 18 has one-third to one-half the risk of developing breast cancer as a woman who had her first full-term pregnancy after age 30. Nulliparous women are also at increased risk. The data suggest that there are stem cells that are involved in the development and differentiation of the immature lobule. Since these cells are “pleuripotential,” they are likely more susceptible to malignant transformation from exposure to carcinogens. Once the lobules have differentiated there are likely fewer stem cells, and the risk from exposure to carcinogens likely diminishes. A full-term pregnancy causes lobular differentiation in preparation for lactation. Consequently, a full-term pregnancy at an early age, resulting in lobular differentiation, may reduce the window of opportunity during which a carcinogen could have an effect on the epithelium. Differentiation takes place over a longer period of time if a pregnancy does not hasten the process. Although an early full-term pregnancy does not eliminate the risk of breast cancer, the rapid completion of lobular development likely narrows the window of opportunity during which the proliferating cells of the terminal duct are more likely to be susceptible to damage from a carcinogen.
There appears to be less agreement on the effect of a pregnancy on a growing cancer. Some data suggest that the prognosis is not influenced by pregnancy (5,6). Other studies suggest that a concurrent pregnancy does worsen the prognosis. Women who are diagnosed with breast cancer during pregnancy tend to present with more advanced disease. In the review by Swinford et al from Memorial Sloan-Kettering Cancer Center in New York, 65% of the women who were diagnosed with breast cancer during or within 1 year of a pregnancy had positive nodes at diagnosis (7). This is a higher percentage than the average population.
Guinee et al, using data from the International Cancer Patient Data Exchange System, evaluated 407 women from the ages of 20 to 29 and found that for women whose cancer was diagnosed during pregnancy, the risk of dying from breast cancer was 3.26 times greater than for a woman who never had children (8). They also found that the risk decreased by 15% for each year between a pregnancy and
the development of the cancer. However, a review by Kroman and Mouridsen suggested that pregnancy itself was not an independent prognostic variable (9). In a study of Swedish women, Baldstrom et al found that women who developed breast cancer during pregnancy had a particularly worsened prognosis (10).
the development of the cancer. However, a review by Kroman and Mouridsen suggested that pregnancy itself was not an independent prognostic variable (9). In a study of Swedish women, Baldstrom et al found that women who developed breast cancer during pregnancy had a particularly worsened prognosis (10).
Delay in diagnosis has been suggested as an explanation for the poorer prognosis associated with pregnancy. Because the breast may become “lumpier” during pregnancy, lesions may be overlooked or dismissed and intervention delayed so that the patients are ultimately diagnosed with more advanced disease. The significance of this delay, however, is unclear. Mann et al found that 58% of women who had palpable cancer (not associated with pregnancy), whose diagnosis was delayed for 2 months or more after the onset of symptoms, had positive nodes, whereas only 18% had positive nodes when diagnosis was made within 2 months of the onset of symptoms (11). The group at Sloan-Kettering, however, did not find this association in their series of pregnancy-associated cancers, and Guinee et al found that the poorer prognosis was only slightly affected by the size and stage of the tumor, suggesting that this was not the only explanation.
A simple explanation might be that if there is a spectrum of breast cancers that start each year for women beginning in their teens or early twenties (some slow-growing, some with intermediate growth rates, and some with rapid growth rates) based on the growth rates alone, the faster-growing cancers will be the first to become detectable, and this might explain the preponderance of these cancers among the youngest women (12). I suspect that the cancers are already growing, but the hormonal milieu of lactation might hasten the growth.
Breast Infection and Abscess
Breast infections are fairly common, particularly during or soon after lactation. The nipple and areola can become dry and chapped, and suckling can cause cracks in the skin through which bacteria can infiltrate. In one study in Australia, 1,193 women were questioned about mastitis and abscess during lactation. In this group, 207 women had mastitis (17%) and 5 women developed a breast abscess (0.4%). For as yet unexplained reasons, we have recently been seeing breast abscesses in nonlactating women. These usually begin as a hot, sore spot in the breast usually but not always near the nipple/areolar complex.
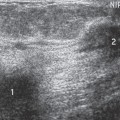
I suspect that many of these are successfully treated clinically so that they do not come for breast imaging evaluation. If the infectious process does not resolve with antibiotic therapy, then the patient is frequently referred for us to determine whether there is a drainable abscess that might be preventing successful antibiotic therapy. Mammography is of little value in these women and is rarely if ever done. Squeezing a breast that contains a pyogenic infection is extremely painful. Even pressure from the ultrasound transducer during sonographic evaluation is very painful. Sonography is the imaging study of choice for evaluating a patient who is thought to have an abscess. If an abscess can be identified and drained, the infection will usually resolve.
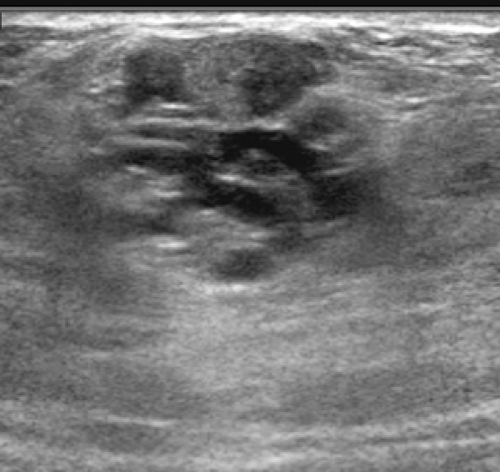
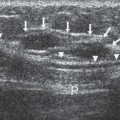
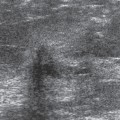
Usually the diagnosis of an abscess is apparent from the clinical presentation. The skin of the breast is very hot and red and thick. The appearance of the skin is similar to what can be seen with inflammatory carcinoma, but cancer is never nearly as painful as a breast infection. It has been described (but it is very rare) that a spontaneous infection occurs in conjunction with a breast cancer. As a consequence, when the ultrasound evaluation shows a hypoechoic structure in the clinical setting of a breast abscess, this is not likely a mass, but rather the abscess. In our experience virtually all breast abscesses are accompanied by marked skin thickening seen on ultrasound (Fig. 18-6). The skin is thickened, and there are collections of fluid between the skin and subcutaneous tissue. We believe that this likely represents fluid-filled lymphatics, all consistent with edema. The problem with a breast abscess is that if the hypoechoic tissues are not clearly fluid-filled, the introduction of a needle or catheter into the presumed collection may yield fluid or just destroyed tissue forming a phlegmon.
The only way to determine whether there is a drainable abscess is to insert a needle and try to remove fluid. An attempt is made to provide some local anesthesia, but this
will likely cause a great deal of pain by itself. I use an 18-gauge or larger needle to enter the presumed cavity while an associate provides syringe suction through collecting tubing coupled to the needle. The needle is repositioned to try to remove all of the fluid if possible. If there is no fluid to be removed, the effort is terminated and the patient returns to clinical care. On rare occasions a patient will return several days later for a repeat effort in the hope that the phlegmon will have liquefied in the interim and can be aspirated. We have on rare occasion placed a pigtail catheter into a large abscess cavity that is defying therapy and this has been associated with success, even though it is never clear whether it was the initial drainage or the constant fluid removal through the catheter that resulted in successful treatment.
will likely cause a great deal of pain by itself. I use an 18-gauge or larger needle to enter the presumed cavity while an associate provides syringe suction through collecting tubing coupled to the needle. The needle is repositioned to try to remove all of the fluid if possible. If there is no fluid to be removed, the effort is terminated and the patient returns to clinical care. On rare occasions a patient will return several days later for a repeat effort in the hope that the phlegmon will have liquefied in the interim and can be aspirated. We have on rare occasion placed a pigtail catheter into a large abscess cavity that is defying therapy and this has been associated with success, even though it is never clear whether it was the initial drainage or the constant fluid removal through the catheter that resulted in successful treatment.
I am unaware of any study that compared randomly assigned women to having imaging-guided abscess evaluation and drainage, to clinically guided drainage. The studies that have been published support the use of imaging evaluation and imaging-guided abscess drainage, but it is unclear whether this has any major benefit over clinical evaluation and drainage.
In one large study Ulitzsch et al reviewed 108 women who were suspected of having a breast abscess. Sonography suggested 56 abscesses. Among these, 23 abscesses that were less than 3 cm were treated by just needle aspiration, while 33 that were larger than 3 cm had a catheter placed under ultrasound guidance. All of the abscesses treated with catheter drainage resolved. One woman whose abscess was just aspirated went on to have surgical incision and drainage. This is the most optimistic of the reports on imaging-guided treatment of breast abscess. Others have had varying success with imaging-guided percutaneous drainage of abscesses (13,14). The available data suggest that if an abscess is to be successfully treated, it should be drained as completely as possible. Cases that went on to require surgical treatment were often incompletely drained.
Breast Biopsy and its Effect on the Breast
When it is not possible to determine whether a lesion is benign or malignant, a biopsy is often indicated. Any interventional procedure in the breast offers the opportunity of affecting the imaging study by causing tissue disruption, a hematoma, or fluid collection. The term “breast biopsy” is a general term that can be applied to anything from the introduction of a 25-gauge needle to a large segmental resection. Breast lesions can be biopsied in multiple ways.
Needle Biopsy
Needle biopsy is the least invasive of the interventional methods used to determine the nature of an abnormality. Needles can be positioned using clinical guidance (when the lesion is palpable) or imaging guidance. Needle biopsies of the breast can be performed using mammography (15), stereotactic mammography (16), ultrasound (17), computed tomography (CT) (18), magnetic resonance imaging (MRI) (19), and radionuclide imaging to guide the positioning of the needle.
Fine-Needle Aspiration Cytology
The least traumatic method of needle biopsy involves the aspiration of cells for cytologic analysis through needles that range from 25 to 20 gauge (termed “fine needles”). The material that is removed consists of cells that have been separated from their surroundings; they are analyzed for their cytologic features. In well-trained hands, fine-needle aspiration (FNA) is extremely accurate in the diagnosis of malignancy. Because cytopathologists tend to be extremely conservative, the reported false-positive rates for FNA are extremely low. As a consequence, however, the false-negative rates (if indeterminate is included) are fairly high, necessitating open biopsy. Furthermore, many series have a significant number of tests in which the gathered material was insufficient for analysis. In most hands it is not possible to differentiate in situ from invasive cancers using FNA.
Core Needle Biopsy
Core needle biopsy has become the preferred method, short of an open biopsy, for making an accurate diagnosis. The needles are much larger than for FNA (18 gauge to 11 gauge or larger). These permit the removal of intact slivers of tissue or even larger samples (the en bloc method) that are analyzed using standard histologic methods. Because the tissue architecture is preserved, invasive cancer can be distinguished from in situ (assuming the appropriate areas of the tumor have been sampled) using the core biopsy technique. Hematomas are fairly common following core needle biopsy. They are usually small and resolve quickly, but we have seen several large hematomas that took many months to resorb. Permanent scarring that is visible on the mammogram after a core biopsy is rare. Clips are frequently placed to mark areas of previous core needle biopsy and are easily seen by mammography.
Surgical Breast Biopsy
The most common form of diagnostic biopsy, and probably the most accurate if performed properly, is an open biopsy performed by the surgeon. This involves an incision through the skin and dissection to remove a volume of tissue containing the abnormality. If the entire area of suspicion is removed, the procedure is termed an “excisional” biopsy. If only a portion of the tissue volume is removed and the lesion is cut into, the procedure is an “incisional” biopsy. The term “lumpectomy” should be reserved for the excision of a malignant process. A lumpectomy involves the removal of the
tumor with an amount of normal surrounding tissue. The goal is to excise a sufficient amount of surrounding normal tissue so that there is no cancer at the margins of the excised tissue. If there is tumor at the margin of the excised tissue, then it is likely that there is still tumor in the breast (the other side of the margin). Free margins reduce (but do not eliminate) the likelihood that there is residual tumor in the breast, so that radiation therapy will have a greater chance of eradicating any residual cells in the breast. Even when a tumor appears to be completely removed, there may still be residual cancer in the breast. Even the pathologist, analyzing the excised tissue, cannot be certain that there is no residual tumor. A study done at Harvard Medical School in which small invasive breast cancers (1 cm or smaller) were excised with free margins by pathologic review, in an effort to avoid irradiation, had to be terminated early because the recurrence rate was too high. Breast cancers recur after lumpectomy because the tumor extended beyond the margins of excision and was not appreciated by the surgeon or the pathologist due to the sampling error inherent in the evaluation of excised tissue. Some surgeons excise the cancer with a rim of what they feel is normal tissue and then excise additional tissue from the walls of the biopsy cavity. These latter tissues are called the “shaved margins.” If there is tumor in the shaved margins, then this indicates that the tumor is likely more extensive than can be appreciated by any tests (including direct surgical observation), and since there is no way to determine its full extent, the patient will likely need a mastectomy.
tumor with an amount of normal surrounding tissue. The goal is to excise a sufficient amount of surrounding normal tissue so that there is no cancer at the margins of the excised tissue. If there is tumor at the margin of the excised tissue, then it is likely that there is still tumor in the breast (the other side of the margin). Free margins reduce (but do not eliminate) the likelihood that there is residual tumor in the breast, so that radiation therapy will have a greater chance of eradicating any residual cells in the breast. Even when a tumor appears to be completely removed, there may still be residual cancer in the breast. Even the pathologist, analyzing the excised tissue, cannot be certain that there is no residual tumor. A study done at Harvard Medical School in which small invasive breast cancers (1 cm or smaller) were excised with free margins by pathologic review, in an effort to avoid irradiation, had to be terminated early because the recurrence rate was too high. Breast cancers recur after lumpectomy because the tumor extended beyond the margins of excision and was not appreciated by the surgeon or the pathologist due to the sampling error inherent in the evaluation of excised tissue. Some surgeons excise the cancer with a rim of what they feel is normal tissue and then excise additional tissue from the walls of the biopsy cavity. These latter tissues are called the “shaved margins.” If there is tumor in the shaved margins, then this indicates that the tumor is likely more extensive than can be appreciated by any tests (including direct surgical observation), and since there is no way to determine its full extent, the patient will likely need a mastectomy.
Changes Seen by Mammography Following a Breast Biopsy
Changes Seen Following a Needle Biopsy
Sickles has shown that the insertion of a needle into the breast, even for a simple aspiration, can produce changes, due to either edema or hematoma, that are evident if a mammogram is performed soon after the procedure (20). It is often better to obtain a mammogram (if one is indicated) before any intervention. If, however, a needle procedure has been performed, it is prudent to wait 2 or more weeks to perform the mammogram so that any spurious tissue changes can resolve. Core needle biopsies frequently result in hematomas (Fig. 18-7), although these appear to resolve fairly rapidly, with little effect noted at 6 months (21).
Changes Seen Following a Surgical Biopsy
It is a commonly repeated but fallacious belief that a surgical breast biopsy for what proves to a benign abnormality results in permanent scarring of the breast that is confusing on future mammograms (22). This belief is, in fact, unsubstantiated. The persistence of architectural distortion, or a spiculated mass, seen on a mammogram a year or more after a breast biopsy with benign results is rare and is even more rarely a problem. Although the breast, as evaluated mammographically, can be a fairly slow organ to heal, there is usually little or no evidence of a biopsy at the time of the next annual screening mammogram after a biopsy with benign results, although postsurgical scar is more evident on DBT.
Early Changes Following a Surgical Breast Biopsy
The only reason to perform a mammogram within days or weeks of surgery is if there is uncertainty that a lesion has been removed. If specimen radiography confirmed that the abnormality was excised and the histology was benign, there is no reason to obtain a mammogram soon after surgery. The only time that an early postoperative mammogram is needed is if there is any question as to whether the targeted lesion was actually excised. There have been cases in which women have had very suspicious lesions on clinical examination and mammography. The clinically guided excision revealed a benign process. An early repeat mammogram (within days to 1 or 2 weeks after the surgery) revealed that the lesion had not been excised, and re-excision revealed malignancy (23). If the biopsy result is concordant with the preoperative diagnosis, then there is no reason to obtain an early postoperative mammogram. Unless there is a change on clinical examination to prompt earlier evaluation, there is no reason to do any more than return to annual screening after a benign breast biopsy. It is very unusual for postsurgical change to present a problem of diagnosis a year or more after the surgery.
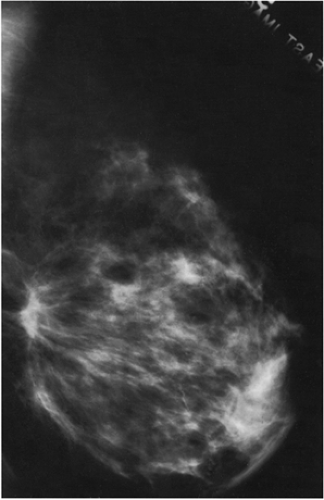
If, however, a mammogram is obtained in the early postoperative period (6 months or less), there may be prominent changes. The breast may show minimal (Fig. 18-8) or even no changes, but some women may develop a hematoma or seroma forming a mass and architectural distortion. This is not that unusual and may be dramatic (Fig. 18-9). Seromas and hematomas are usually indistinguishable. They are both radiodense, although it is likely that a fresh hematoma is more attenuating than a seroma due to its iron (heme) content. The margins may be well circumscribed or ill defined. The reaction and fibrosis associated with healing may even cause the margin to be spiculated (Fig. 18-10).
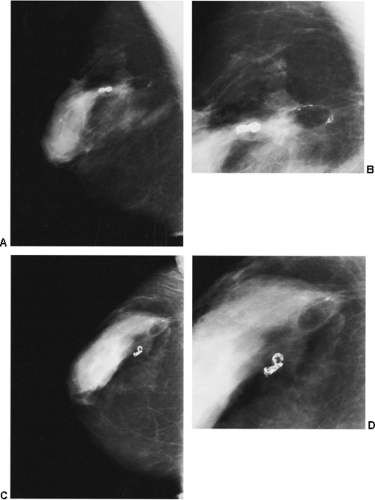
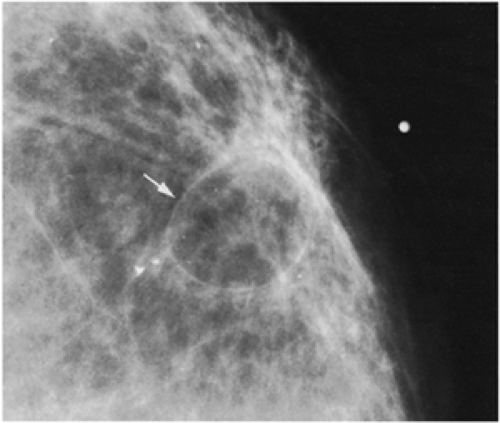 Figure 18-11 This posttraumatic oil cyst (arrow), a form of fat necrosis, formed after a benign breast biopsy. |
Fat necrosis may result from surgery. This is easily diagnosed when an encapsulated, fat-containing lesion (Fig. 18-11) or large calcifications develop in the bed of the surgery (Fig. 18-12). The time of development of fat necrosis is variable, ranging from months to years.
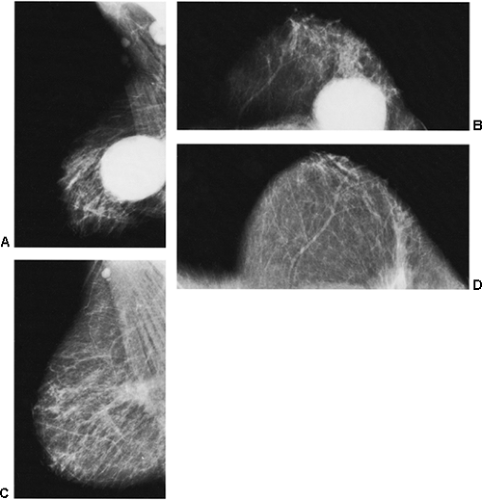
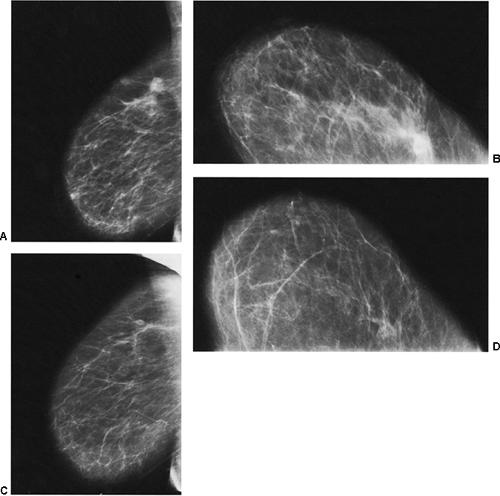
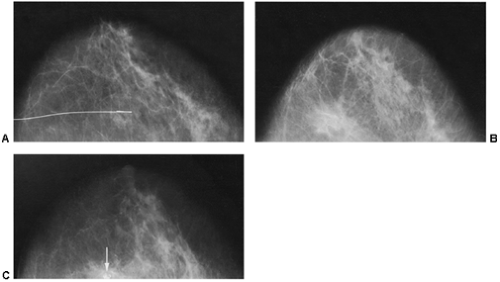
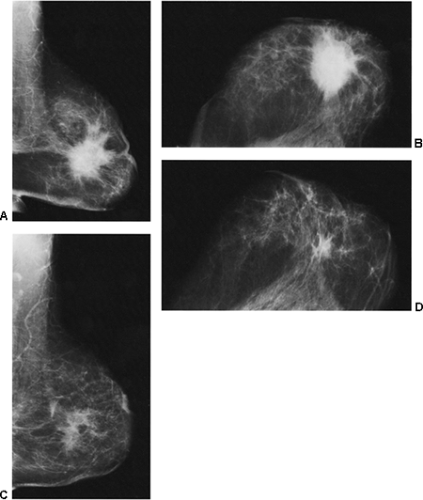
If there is any question raised by the findings from a mammogram obtained within a year after surgery for a benign lesion, the pathology of the excised tissue should be reviewed to be certain that it was truly a bland benign lesion. Logic should prevail. If the preceding biopsy in fact removed the lesion and the tissue that was excised was clearly benign, it is extremely unlikely that a cancer was present in the surgical bed and missed by the pathologist and developed over a few months into a large, visible breast cancer. Thus, when a mammogram is obtained in the early postoperative period after a biopsy with benign results and a spiculated lesion is present, it is virtually certain that it is only postoperative change, and only short-interval follow-up is suggested to monitor its resolution (Fig. 18-13A–F).
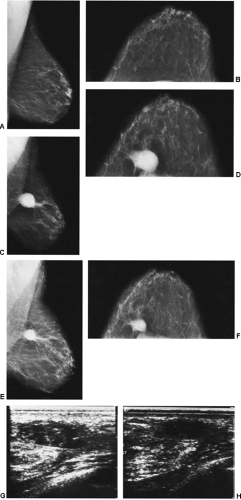
We have found ultrasound of little value in assessing these women. The interpretation of ultrasound in the postoperative setting is difficult (see Fig. 18-13G,H). Any postoperative change should remain stable, improve, or resolve. If its appearance worsens by imaging, then intervention may be indicated.
In the immediate post-biopsy period (days to weeks) a hematoma is not unusual (Fig. 18-14). Postsurgical changes may be visible up to a year or more after a biopsy that had benign results. For most women, however, the breast will ultimately heal with little or no visible residual seen on the mammogram (Fig. 18-15). Since the location of a previous biopsy in the breast is usually known, any slight distortion is usually easily accounted for. I have seen two cases where a cancer, coincidentally, developed many
years later near an area of a previous biopsy. The post-biopsy scar made it difficult to appreciate the developing malignancy. Fortunately, this is extremely rare. However, if spiculated architectural distortion was not present on a mammogram subsequent to a benign biopsy, but it develops following studies where no architectural distortion is evident, then it should be carefully evaluated. Postsurgical changes should be evident immediately after surgery and then resolve over time. It would be unlikely for them to develop years after the biopsy.
years later near an area of a previous biopsy. The post-biopsy scar made it difficult to appreciate the developing malignancy. Fortunately, this is extremely rare. However, if spiculated architectural distortion was not present on a mammogram subsequent to a benign biopsy, but it develops following studies where no architectural distortion is evident, then it should be carefully evaluated. Postsurgical changes should be evident immediately after surgery and then resolve over time. It would be unlikely for them to develop years after the biopsy.
In 1981, Sickles and Herzog (23) reported that a persistent, spiculated abnormality after a biopsy with benign results is extremely rare. In their review of women who had had benign breast biopsies, only 3% of women had persistent distortion 2 to 3 years after surgery. These results occurred despite the fact that their data were accumulated at a time when larger volumes of tissue were routinely removed. Mitnick et al found a similar percentage (24). There is likely a variation in the amount of persistent change that depends on the extent of the surgery and the postoperative course
(hematoma formation or infection), but it is extremely rare for postoperative change to be apparent or confusing when the biopsy was benign.
(hematoma formation or infection), but it is extremely rare for postoperative change to be apparent or confusing when the biopsy was benign.
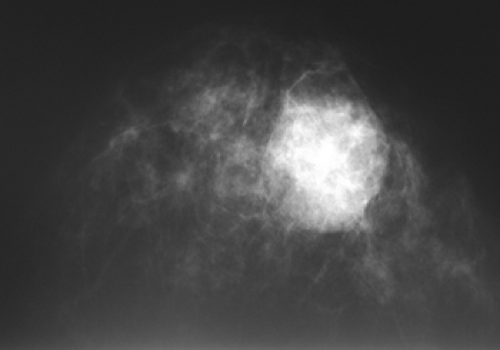 Figure 18-14 This large, radiographically dense mass was merely a postsurgical hematoma 1 month following a benign breast biopsy. |
We performed two studies—a prospective evaluation as well as a retrospective analysis of the mammograms of women who had undergone a breast biopsy with benign results—to determine how often postsurgical change caused a problem in mammographic interpretation. In the retrospective review we identified 58,538 mammographic examinations among 31,025 asymptomatic patients who had routine mammographic screening studies between 1993 and 1996. There were 53,510 studies among patients who had no history of breast biopsy, while 5,028 (9%) were of patients who had a history of having had a previous breast biopsy for benign changes. Among the 53,510 studies done in patients who had not had a previous biopsy, 3,296 (6%) were recalled for additional imaging. Among women who had a previous benign biopsy, 360 (7%) of the 5,028 studies done led to recall for additional imaging. Only eight of the latter recalls for further imaging (0.16%) among the 5,028 studies in women with a prior biopsy for benign disease were related to the biopsy site.
In our prospective study, radiologists reviewed the mammograms of 1,997 consecutive patients presenting to the screening center; 173 of these women reported a prior breast biopsy for benign disease. The radiologists determined how often evidence of a biopsy site was apparent on the mammogram and how often such changes necessitated additional imaging. They found that 24 (14%) of the 173 women who had a prior biopsy for benign disease had mammographic evidence of the biopsy site. Nine (5%) of the 173 women who had previously had a biopsy for benign disease and 86 (5%) of the 1,824 patients without a prior biopsy were recalled for additional imaging. None of the women were recalled because the previous breast biopsy
for benign disease led to confusion or diagnostic concern. We concluded that changes from previous excisional biopsies for benign breast problems are quite uncommon and rarely pose a diagnostic dilemma in the interpretation of routine screening mammograms (25).
for benign disease led to confusion or diagnostic concern. We concluded that changes from previous excisional biopsies for benign breast problems are quite uncommon and rarely pose a diagnostic dilemma in the interpretation of routine screening mammograms (25).
Because postsurgical changes may take several months to resolve, we prefer to avoid the term “scar” until it is clear that the changes have become fixed. We prefer to use the terms “postsurgical” or “posttreatment changes.”
New Baseline Mammogram After a Biopsy With Benign Results
Unless there is a specific reason to obtain a mammogram following a biopsy with benign results, there is no reason to do this routinely. If needed, an early post-biopsy mammogram can be obtained within days of the surgery, as long as compression is carefully tailored to avoid hurting the patient and to avoid causing a wound dehiscence. The only time this type of study is warranted is if there is suspicion that the targeted lesion has not been removed. This may be obvious after needle localization when the specimen does not contain the abnormality, but it can also happen when a palpable abnormality is biopsied (26). If a lesion has not clearly been excised, then an early follow-up study is indicated since a repeat biopsy may be needed.
There are some, however, who advocate an early postsurgical follow-up mammogram even when the results of the biopsy are benign. This is done to assess the extent of tissue disruption or hematoma formation from the surgery so that on subsequent mammograms its progression to healing will be reassuring (rather than seeing change for the first time a year after the surgery). We do not advocate this approach, because postoperative change is rarely visible a year after the surgery when the patient resumes her screening program, and it is even less often a problem. The practice could be criticized for resulting in a significant number of unnecessary extra mammograms. For the few women who have a confusing mammogram at the first screening after a benign biopsy, short-interval follow-up (at 6 months) to monitor the area for resolution or stability will limit the overall number of women having additional studies and radiation exposure.
Extensive Surgery Can Lead to Persistent Change
On rare occasion, the breast heals with a persistent area of architectural distortion following the removal of normal or benign tissue. This is especially true if surgery has been extensive, such as with a reduction mammaplasty (Fig. 18-16). Fat necrosis occasionally occurs, but the lucent-centered posttraumatic oil cyst that may form is easily diagnosed as a benign phenomenon (Fig. 18-17). The large, lucent-centered calcifications of fat necrosis (Fig. 18-18) or dystrophy are rarely confused, morphologically, with those produced by breast cancer. Even less common is the development of a seroma, which is likely an exudative process. On rare occasions these persistent fluid collections arise and can be difficult if impossible to eliminate (Fig. 18-19). They are seen each year by mammography.
Late Changes After a Surgical Breast Biopsy
As noted earlier, persistent postsurgical changes and noticeable architectural distortion that may be spiculated are unusual after a benign breast biopsy. In a few women, however, these changes persist for many years and presumably become a fixed scar. Some observers have suggested that this is more common if a hematoma formed postoperatively, but this has never been scientifically demonstrated. If this type of distortion causes concern, it is helpful to review the mammograms from before the surgery to determine the location of the biopsy within the volume of the breast tissue. It may also be a good idea to review the pathology of the biopsy to be sure that there was no mistake and to be sure that there were no atypical changes that might indicate the possibility that the present architectural distortion is due to malignancy.
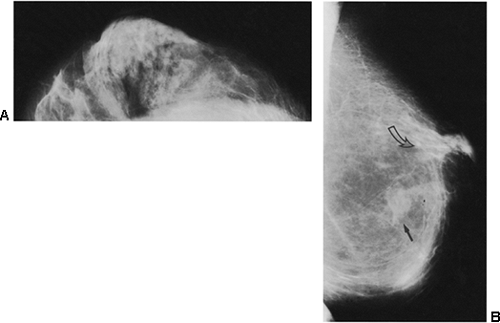
When postsurgical changes do persist after a biopsy with benign results, they can be dramatic (Fig. 18-20). High-contrast mammograms overpenetrate the skin, but a careful evaluation of the incision site may reveal some skin thickening or retraction months to years after surgery. These can be seen more easily on digital mammography due to the equalization routines and the ability to adjust the window and level. Usually subtle (Fig. 18-21) but occasionally striking architectural distortion may be present in the area of excision. There is rarely an associated mass.
After architectural distortion, fat necrosis is probably the most common long-term change caused by surgery. This may result in the development of an oil cyst with a thin wall that encapsulates gelatinized, radiolucent fat within, or the process may produce large, lucent-centered calcifications. The architecture in the region of the biopsy may be distorted, and on occasion, when a large amount of tissue has been excised, the loss may be apparent on subsequent mammograms. Asymmetric breast tissue may be suggested on the contralateral side when the appearance is merely due to the excision of tissue on the ipsilateral side producing the asymmetric appearance (Fig. 18-22) (27).
With the exception of the immediate postoperative period, any changes should become stable or improve with time. If the changes appear to be worsening, then intervention (biopsy) may be indicated.
Marking the Skin Incision
If there is any question as to whether architectural distortion is the result of postoperative change, the relationship of the distortion to the skin incision can be assessed by placing markers (BBs or wires) on the skin scar. Many technologists
are told to do this routinely, but this is not the standard of care. Because confusion over postsurgical change is rare in our practice, we place markers on the skin incision only if there is a question to be resolved. Because the skin incision is frequently at a distance from the site of tissue removal, it is often more useful to compare the present study to the preoperative mammograms that showed the lesion and its location before its removal. This better defines the area where architectural distortion would be expected from the surgical tissue removal.
are told to do this routinely, but this is not the standard of care. Because confusion over postsurgical change is rare in our practice, we place markers on the skin incision only if there is a question to be resolved. Because the skin incision is frequently at a distance from the site of tissue removal, it is often more useful to compare the present study to the preoperative mammograms that showed the lesion and its location before its removal. This better defines the area where architectural distortion would be expected from the surgical tissue removal.
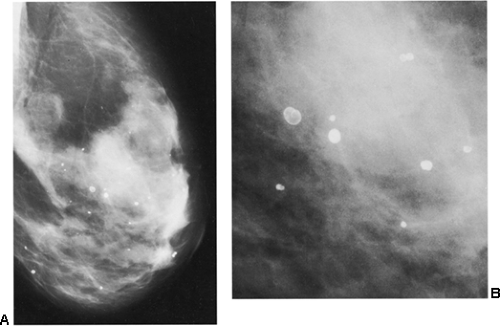 Figure 18-18 Lucent-centered calcifications (A) are always benign. They are likely to be due to fat necrosis. (B) Photographically enlarged. |
Postsurgical Change: A Different Appearance on the Two Projections
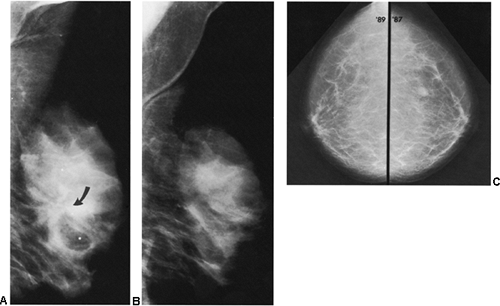
Another helpful differential observation lies in comparing a suspicious abnormality on the two projections. Breast cancer usually looks similarly worrisome on the two views (CC and MLO). When cancer appears as a mass with spicules or as spiculated architectural distortion, it frequently has a similar appearance in both projections. A scar or postsurgical change often has an ominous appearance on one projection but is almost imperceptible in the other projection (Fig. 18-23). Although this is not a definitive observation, it is useful for differentiating postsurgical change from neoplasia when found in association with the other factors that suggest postsurgical change.
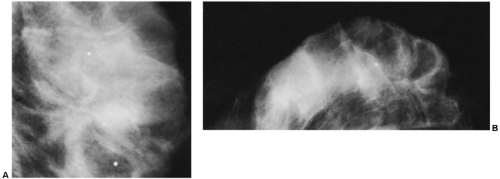 Figure 18-23 Postsurgical change is usually more prominent on one projection than on the other. Three months after surgery, in the same patient as in Figure 18-15, there is postsurgical architectural distortion that is more evident on the MLO (A) projection (photographically enlarged) than medially on the CC view (B). |
Reducing the Likelihood of Postsurgical Changes
Increasing attention has been paid by surgeons to biopsy technique, and this has resulted in an improvement in the cosmetic results after biopsy and less permanent changes on the mammogram than may have occurred in the past.
When a biopsy is performed for a nonpalpable lesion detected by mammography, accurate preoperative localization should be performed. Guides can and should routinely be placed within 5 mm of the lesion (28). Any farther away should be unacceptable. Surgeons should be encouraged to excise the minimum amount of tissue necessary when performing a diagnostic biopsy when cancer is not anticipated.
Some surgeons suggest that postsurgical tissue distortion can be reduced by not approximating the walls of the biopsy cavity with sutures, so that the tissues fill in with less defect than if the walls are sutured together. Suturing the walls of the excision bed may lead to tissue distortion from the contraction of the scarring process and may result in a pronounced defect that forms a depression in the parenchyma, evident on clinical examination. Many breast surgeons use only subcuticular sutures, allowing the tissues to fall together naturally. If meticulous hemostasis has been achieved during the biopsy, there is little postoperative abnormality evident.
Calcifications After a Breast Biopsy With Benign Results
It is fairly unusual for calcifications to form after a breast biopsy with benign results. Although large, lucent-centered calcifications can develop if fat necrosis occurs, this is fairly uncommon unless there has been a large surgical procedure such as a reduction mammaplasty. Suture material can calcify, but this too is rare when a biopsy reveals a benign process. As noted in the following section, suture calcifications appear to be related to more extensive surgery, such as reduction mammaplasty, or the delayed healing after radiation therapy.
Mammography After Reduction Mammaplasty
The most extensive breast surgery (short of a mastectomy) undertaken for benign conditions is the removal of large amounts of tissue to reduce the size of the breast. Reduction mammaplasty is usually performed for cosmetic and self-image purposes or because large breasts can be physically debilitating. Their weight can result in chest wall pain as well as back pain. Grooves can be worn in the individual’s shoulders from the excessive weight of the breasts pulling on bra straps. Reduction mammaplasty may also be performed on the contralateral side after a mastectomy and breast reconstruction to produce more symmetric breasts.
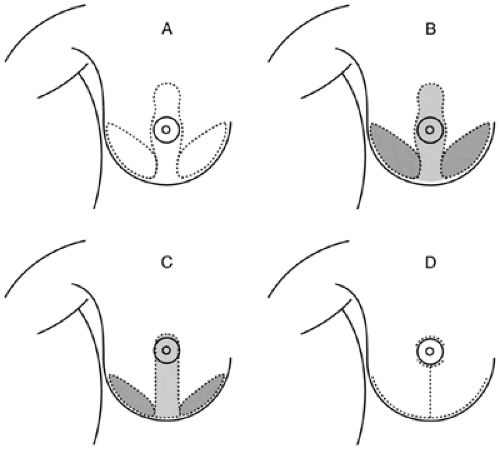
There are several ways to reduce the volume of breast tissue while preserving cosmesis. The most common procedure involves a long incision along the inframammary fold and incisions extending from it to the 6 o’clock edge of the areola and then around the areola. Tissue is removed from the bottom of the breast and between the vertical incisions. The nipple/areolar complex is moved up into a keyhole extension of the vertical incisions, which are then brought together in the midline to reform the smaller breast (Fig. 18-24).
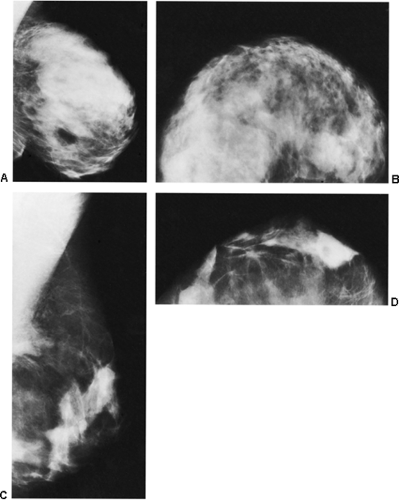
The removal of hundreds of cubic centimeters of breast tissue can produce dramatic changes on the mammogram, or they can be fairly unremarkable and not even noticeable. As might be expected, the fibroglandular tissues can appear redistributed from the usual upper breast location to the newly formed lower breast (29). Nonanatomic parenchymal bands and scars have been described. In our experience, there is often a swirling configuration to the remolded tissues (Fig. 18-25A), and isolated islands of breast tissue can be seen (Fig. 18-25B). Fat necrosis is relatively common with the extensive surgery involved, and oil cysts (Fig. 18-26) as well as benign calcifications can be seen after the surgery. On occasion, presumably as a result of delayed healing, suture calcifications are seen after reduction mammaplasty (Fig. 18-27). Despite the frequently dramatic
changes that may be seen, in some individuals it is difficult without previous films to tell that a reduction procedure has been performed (Fig. 18-28).
changes that may be seen, in some individuals it is difficult without previous films to tell that a reduction procedure has been performed (Fig. 18-28).
Subcutaneous Mastectomy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree