ABSTRACT
Hepatocellular carcinoma (HCC) is the most common form of hepatic malignancy, with high mortality rates recorded globally. Early detection through clinical biomarkers, medical imaging and histological assessment followed by rapid intervention are integral for positive patient outcomes.
Although contrast-enhanced computed tomography scans and magnetic resonance imaging are recognised as the reference standard for the diagnosis and staging of HCC in international guidelines, ultrasound (US) examination is recommended as a screening tool for patients at risk. Contrast-enhanced US (CEUS) elevates the standard of an US examination using US contrast agents (UCAs), capable of diagnosing focal liver lesions with high efficacy. Most UCAs are purely intravascular, offering clinicians a dynamic representation of a lesions’ arterial phase vascular kinetics, which is seldom seen in such detail during computed tomography or magnetic resonance imaging assessments. Despite its benefits, there is incongruity between international societies on the role of CEUS in the HCC clinical pathway. The transient nature of pure blood-pool agents is suggested to be insufficient to justify CEUS as a primary modality due to the inability to consistently perform whole liver imaging, alongside disputes regarding its capabilities to differentiate HCC from intrahepatic cholangiocarcinoma.
A sinusoidal phase UCA affords clinicians the opportunity to perform whole liver imaging through Kupffer cell uptake in addition to visualising lesion vascular kinetics in the arterial and portal venous phases. Therefore, the purpose of this review was to examine the role of CEUS in the HCC clinical pathway in its current practice and observe how a Kupffer cell sinusoidal phase UCA may supplement contemporary diagnostic techniques through a multi-modality, multi-agent approach.
Introduction
Hepatocellular carcinoma (HCC) remains the most prevalent form of liver cancer and ranks as the third highest cause of cancer-related deaths worldwide, where early and effective diagnosis plays a pivotal role in patient prognosis [ , ]. Ultrasound (US) examination serves as a common imaging modality in the assessment of focal liver lesions, including the screening of patients at risk of HCC [ ]. However, despite its widespread popularity, US detection of HCC on a cirrhotic background is challenging due to both US attenuation in steatotic parenchyma and the appearance of very coarse liver echotexture, which may impair the identification of small tumours [ ]. Imaging with ultrasonography may also be restricted in its ability to perform whole liver imaging in patients with limited insonation windows and due to relatively low contrast resolution [ , ]. Contrast-enhanced US (CEUS) improves the diagnostic capabilities of an unenhanced US examination, being widely adopted due to its easy access as a complement to B-mode ultrasonography, offering real-time imaging providing detailed vascular kinetics and its cost effectiveness [ ]. However, it remains listed as a second-line imaging modality for HCC diagnosis as underlined by the European Association for the Study of the Liver ( Fig. 1 ) and the American Association for the Study of Liver Diseases in a threefold manner, due to an insufficiency at providing a whole overview of the liver parenchyma as their in vivo life-span is too short lived, a lack of comparability to computed tomography (CT)/magnetic resonance imaging (MRI) due to the operator dependency of ultrasonography and the controversy surrounding the capacity of CEUS to differentiate between HCC and intrahepatic cholangiocarcinoma (ICC) [ ].

Current image interpretation guidelines for HCC diagnosis using CEUS are tailored to the transient nature of the more commonly available purely intravascular agents [ ], providing the opportunity to explore whether an agent with sustained in vivo stability and a unique post-vascular phase [ ], Sonazoid (GE HealthCare, Amersham, UK), may supplement the value of UCAs in HCC diagnostics. Such specific post-vascular imaging features presented by Sonazoid could be compared with the additional information provided by hepatobiliary (HPB)-specific MRI agents providing an incremental diagnostic added value for HCC versus extra-cellular MRI agents.
Materials and methods
The aim of this review was to identify the role of CEUS in imaging patients with regard to the current clinical pathway for oncological imaging of HCC and explore how the sinusoidal phase UCA, Sonazoid, may supplement the pathway further through its specific pharmacokinetic properties. A literature search was conducted using the National Library of Medicine database (pubmed.ncbi.nlm.nih.gov) to gather publications on current national guidelines regarding HCC management, conduct of a hepatic CEUS procedure outlined by clinical luminaries, current image interpretation methodologies using CEUS, the pathophysiology of HCC and the clinical utility of the UCA Sonazoid in imaging patients suspected or known to have HCC.
Search terms were adapted depending on the relevant commentary section of this review, whereas truncation and parentheses were combined with Boolean operators to ensure a breadth of literature was synthesised [ ]. For establishing current clinical management guidelines for HCC and hepatic CEUS practice procedures, the following search terms were used: (liver OR hepatic) AND (HCC OR hepatocellular carcinoma) AND clinical practice AND (guidelines OR management) AND (diagnos* OR treatment) AND (European OR American OR Asian); (liver OR hepatic) AND (CEUS OR contrast enhanced ultrasound) AND clinical practice AND (guidelines OR recommendations).
For the image interpretation methodologies of HCC assessment with CEUS, the following search terms were used: (liver OR hepatic) AND (HCC OR hepatocellular carcinoma) AND diagnos* AND imaging AND (CEUS OR contrast enhanced ultrasound) AND li-rads AND (image interpretation OR reporting). For signifying the pathophysiology of hepatocarcinogenesis and how this affects diagnostic imaging, the following search terms were used for a broad overview: hepatocarcinogenesis AND (radiology OR imaging) AND (histology OR histopathology OR pathology), whereas the following terms were used for a focused assessment concerning Kupffer cell distribution through the hepatocarcinogenic process: (HCC OR hepatocellular carcinoma) AND hepatocarcinogenesis AND Kupffer cell.
For the physicochemical characteristic properties and clinical applications of Sonazoid in HCC imaging, the following search terminology was used for a broad outlook: (sonazoid OR perfluorobutane OR perflubutane) AND (properties OR characteris*); (sonazoid OR perfluorobutane OR perflubutane) AND (HCC OR hepatocellular carcinoma) AND diagnos*, whereas specific literature pertaining the Kupffer phase was produced in a supplementary search when including: (Kupffer OR post-vascular).
Literature was included for comment if published by national societies with key working groups on hepatic neoplasia imaging, working groups responsible for the publication of recognised clinical practice guidelines in HCC management, and/or literature examining the use of UCAs/Sonazoid for imaging treatment-naïve neoplasms of hepatocellular origin. The literature must also have been published as a form of primary (e.g., clinical trial, randomised control trial, comparative study) or secondary (meta-analysis, review, or systematic review) research. Literature examining neoplasms of non-hepatocellular origin or post-treatment imaging were excluded for comment in this review. References within the literature identified during the literature search were also screened for relevance and eligibility for comment in this review.
Results
A total of 1425 publications were produced from the literature searches. After the removal of duplicates, studies with limited applicability to the reviews topic, articles that were unavailable or not published in the English language and those that further met the reviews exclusion criteria, a total of 93 publications were eligible for possible inclusion throughout this review. Figure 2 outlines the broad literature identification process.

Discussion
Liver CEUS in practice
Guidelines from the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) describe a CEUS liver examination as comprising three overlapping vascular phases after the injection of the UCA, followed by a post-vascular phase dependent on the UCA used ( Fig. 3 ) [ ]. The vascular phases concern the liver’s dual vascular supply, that is, hepatic artery and portal vein (25%–30% and 70%–75% of liver haemodynamics in non-cirrhotic conditions, respectively) and their influx timings:
- •
Arterial phase – Indicates the extent and pattern of a focal liver lesions arterial blood supply.
- •
Portal phase – Demonstrates the perfusion of UCA in normal hepatic parenchymal tissue through the portal system.
- •
Late/sinusoidal phase – Displays remnant circulation of the UCA until it is cleared from circulation, depending on the type and dose of UCA, total scanning time, sensitivity of the US system, system settings, patient habitus and acoustic conditions.
- •
Post-vascular/Kupffer phase – Represents Sonazoid’s uptake by phagocytotic cells of the reticuloendothelial system, such as, Kupffer cells, and can last up to 2 h post-injection.

Established clinical interpretation guidelines
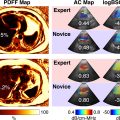
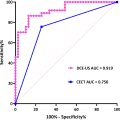
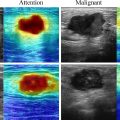
According to the American College of Radiology’s Liver Imaging Reporting & Data System (LI-RADS) and the EFSUMB guidelines on liver CEUS imaging, HCC detection is dictated by the presence of non-rim, non-globular hyperenhancement in the arterial phase, with late and mild venous washout [ ] ( Figs. 4 and 5 ).


Arterial phase hyperenhancement (APHE) is reported to be homogenous and intense in typical HCC, although larger and poorly differentiated (p-Diff lesions may demonstrate heterogenous enhancement due to intra-nodular cellular necrosis [ ]. Rim, or peripheral discontinuous or globular hyperenhancement, is atypical for HCC, with rim APHE most often associated with non–hepatocyte-derived intrahepatic malignancies such as ICC or metastatic deposits and peripheral, discontinuous or globular APHE associated with haemangiomata [ ]. Although general guidance on washout for HCC is defined by its mild occurrence at least 60 s after the UCA bolus, some discrepancies are observed in the literature. Terzi et al. [ ] reported late and mild washout in 97% of HCCs in their large retrospective study. However, it is acknowledged that the degree and timing of washout also depends on the HCC differentiation grade, with reports of rapid washout being observed in p-Diff HCC, and slow or no washout observed in well-differentiated HCC [ ]. This washout atypia is thought be observed in up to 50% of cases [ ]. Such variance observed in HCC imaging with CEUS highlights that possible differences in patient demographics and lesion characteristics may affect the global generalisability of current image interpretation guidelines if based solely on the transient nature of pure blood pool UCAs.
Through phagocytosis by Kupffer cells, Sonazoid offers the opportunity to scan the entire liver 10 min or more after its administration. From that perspective, the Asian Federation and Society of Ultrasound in Medicine and Biology advocates for the use of a dynamic imaging technique based on patterns observed in both the vascular and post-vascular phases, known as defect reperfusion imaging [ ]. This multi-parametric approach takes advantage of the morphological features of typical hepatic malignancies, notably being a distinct vascular pattern and a sparsity of intra-nodular Kupffer cells [ ]. The pharmacokinetics of Sonazoid may, therefore, facilitate whole liver imaging with US in a unique fashion through combination of B-mode imaging for initial lesion detection and assessment, imaging a lesion’s vascular kinetics in the dynamic vascular phase, with additional lesion detection and assessment during the post-vascular phase (Fig. 6) . If additional or occult nodules are identifiable through the post-vascular phase, a repeat injection with any UCA can provide additional diagnostic information. Early primary research [ ] has been undertaken examining this application in the intraoperative management of liver metastases, concluding that Sonazoid aided in identifying occult lesions. Additional projects could be conducted to examine such a use case closely considering hepatic CEUS will continue to be limited by factors that affect B-mode ultrasonography, such as patient habitus, excessive bowel gas, lesions in anatomically challenging segments and equipment-specific limitations.

To date, much of the image interpretation guidelines originating from Europe and the Americas is based on the vascular kinetics of pure blood pool UCAs. This is reflected by the American College of Radiology guidelines’ omission of the Kupffer phase when describing how to perform a CEUS examination of the liver ( Fig. 7 ). However, such omission is not unexpected, given the current lack of commercial licensing for Sonazoid in the United States or the European Union, and much of the data originating in countries where Sonazoid is licensed, primarily in East Asia.

Following a combination of a systematic literature review and additional comprehensive local primary research, the use of UCAs with specific physicochemical characteristics offering a unique, physiological paradigm to hepatic imaging may be reflected in revised LI-RADS criteria and/or international image interpretation and clinical management guidelines. Such indications may eventually be comparable with the use of physiological MR agents, where gadoxetic acid or gadobenate dimeglumine provide clinicians with ancillary findings favouring malignancy or benignity through hepatocyte uptake [ ].
Morphological features of HCC
The formation of HCC is recognised as a complex stepwise process of neoplastic hepatocarcinogenesis, linked with advanced intrinsic liver disease [ ]. Initially, regenerative nodules form within the hepatic parenchyma, which may progress to low-grade dysplastic nodules before high-grade dysplastic nodules, which then undergo malignant de-differentiation into the progressive HCC subtypes: early HCC, well-differentiated HCC, moderately differentiated (m-Diff) HCC, and p-Diff HCC [ ] ( Fig. 8 ).

As dysplastic nodules undergo malignant transformation and de-differentiation, there is a gradual transition from the normal dual arterio-portal blood supply to a purely arterial supply. A reduction in native hepatic arterial flow precedes reduced native portal venous flow, before advanced HCC become supplied purely by newly formed unpaired arteries due to carcinogenic neo-angiogenesis [ ]. These haemodynamic changes contribute to the varied multi-modality appearances of hepatocellular neoplasms undergoing malignant de-differentiation. Progressed HCC lesions depict the characteristic non-rim, non-globular APHE with washout observed in the sinusoidal phase due to hepatic parenchyma receiving additional enhancement from the portal venous system, whereas the tumours unpaired arterial supply drains directly into sinusoidal tracts or the peri-nodular portal venous system, depending on the stage of hepatocarcinogenesis [ ]. Early HCCs pose more of an interpretational challenge as their enhancement dynamics depend on the extent of changes in vascular morphology. It is not uncommon for such lesions to iso- or hypo-enhance in the arterial phase due to an initial reduction in native arterial blood supply without neo-arteriogenesis, which then may iso-enhance in the portal phase due to preserved portal venous blood perfusion from portal triad tracts remaining in situ ( Fig. 9 ) [ ].

Differences in the characteristics specific to both the patient and UCA may contribute to a degree of variance when considering these haemodynamic patterns [ ]. For pure blood pool agents, the intensity and timing of enhancement may depend on the in vivo stability of the micro-bubble and the extent, volume and distribution of vascular perfusion in both the focal lesion and the entire liver [ ]. To ensure optimal enhancement performance, specific US system settings are required for a given dosage of an agent, both of which remain heavily dependent on its volume concentration, gas core and/or shell viscoelasticity [ ]. Micro-bubbles with increasingly elastic shells require lower acoustic pressures to incite a sufficient non-linear oscillation [ ]. Moreover, the lower the molecular weight of the gas core an agent uses also results in a decreased in vivo lifespan owing to increased gaseous solubility in the blood, which decreases as the gas’s molecular weight increases [ ]. Such factors may contribute to an element of pseudo-washout artifact due to micro-bubble dissolution or destruction rather than an actual washout event having occurred; hence, the skill and knowledge of the operator is key during a CEUS examination [ ].
As with other UCAs, Sonazoid cannot extravasate past the vascular endothelium due to its size, leading to comparable imaging performance(s) in the arterial and portal venous phases, whilst the agent remains purely in the blood pool [ , ]. However, due to the use of perfluorobutane as its gas core and stabilising phosphatidyl serine outer shell, it offers sustained in vivo circulation times and a unique ability to elucidate the absence or presence and distribution of Kupffer cells into the diagnostic assessment due to micro-bubble phagocytosis [ , ]. Kupffer cells are reticuloendothelial system macrophages lying within the lumen of hepatic sinusoidal capillaries, adherent to the endothelial wall. Serving as integral components of the immune system, their primary purpose in healthy individuals is to filter the blood of foreign bodies, endotoxins and other microbial contents originating within the gastrointestinal tract via the portal venous system through phagocytosis [ ] ( Figs. 10 and 11 ).