KEY POINTS
A fetal neuroscan is not complete without a thorough examination of the orbits, the eyes, and their surroundings.
Malformations of the eyes can be solitary or in association with other anomalies. Therefore, a search for those should be undertaken.
Any alteration in the shape or size of the orbits, the interorbital distances should be evaluated as a possible sign of other anomalies.
Whenever fetal position enables, transvaginal sonography should be used.
The ultrasonographic examination of the eyes is an important and integral part of the fetal face survey. This chapter describes the methodology of the orbit and eye evaluation, the ultrasonic landmarks of the normal eye, and the features of congenital abnormalities that may be detected in the fetus. The presented data are a summary of the English literature on this topic, as well as the authors’ experience.
Congenital blindness is a common disorder in developing countries in contrast to Western countries.1,2,3,4 Robinson and coworkers1 reported a prevalence of 3 per 10,000 births for eye malformations in British Columbia. Stoll and associates4 found congenital eye malformations in 7.5 per 10,000 births in France. Studies in Great Britain5,6 have shown that about half of the cases of childhood blindness are genetically determined. Twenty percent of all cases were autosomal dominant, 17% autosomal recessive, 5% X-linked, and 8% were thought to be multifactorial.5 Intrauterine infections such as rubella and toxoplasmosis are also regarded as major factors contributing to eye malformations. More recently, fetal alcohol syndrome has become well recognized as a cause of ocular abnormalities, particularly of optic nerve hypoplasia.5,7
The most commonly described ocular abnormalities were cataract (30%), microphthalmia (24%), coloboma (9%), and anophthalmia (4%).1,4,5 Robinson et al1 and Phillips et al6 also reported that cataract was the most common eye abnormality associated with congenital blindness.
As extending the previous study performed in France, Stoll et al,7 reporting on 212,479 deliveries, found a slightly lower prevalence of congenital eye malformations of 6.8 per 10,000. In this study, the prevalence of cataract was 2.7/10,000; of microphthalmia, 1.7/10,000;7 of anophthalmia, 0.23/10,000; and of coloboma, 1.4/10,000.5 Associated fetal anomalies included clubfeet, microcephaly, hydrocephaly, cleft lip and palate, and facial dysmorphism.7 The affected neonates were smaller, weighed less, had smaller head circumference and lower placental weight, and were more often complicated by threatened abortion or oligo- or polyhydramnios than controls.7 Their mothers more often used drugs during pregnancy, and their fathers were more often exposed to occupational hazards than fathers of controls.7 Eye malformations were associated with parental consanguinity.7 The recurrence risk for first-degree relatives of probands was 8.9%;7 this risk was more than 3 times that for additional, nonocular malformations.7
Genetic counseling of the affected families is of utmost importance. The genetics of several congenital malformations with eye abnormalities is known (eg, cataract Coppock-like, Lowe syndrome, Norrie disease, X-linked retinitis pigmentosa, chorioderemia, and retinoblastoma).1,8,9 In these cases, early prenatal diagnosis is therefore possible.
The importance of ultrasound (US) in the prenatal detection of eye abnormalities is discussed later in this chapter.
Figure 14–1 depicts the developmental process of the embryonic and fetal eye. Ages here are given as postconceptional days and weeks, for the first few weeks, then later on as postmenstrual weeks. The eyes first appear in the 22-day-old embryo as a pair of lateral grooves that originate from the neural fold of the forebrain and form the optic vesicles.10,11 Subsequently, the optic vesicles invaginate and form the optic cup.
Figure 14–1.
Development of the eye from day 21 to day 40 (postconception). (From Larsen, 2001,11 with permission.)

The lens vesicle originates from the surface ectoderm at 33 days and subsequently differentiates into the lens at 39 to 47 days. Both the developing lens and the retina are vascularized by the hyaloid artery.
The optic cup is connected to the brain by the optic stalk. The nerve fibers that emerge from the retina are connected with the brain through the optic stalk, which develops into the optic nerve during gestational week 8. At the end of the fifth week, the optic capsule is completely surrounded by a loose mesenchyme. This tissue differentiates into an inner layer comparable to the pia mater of the brain and an outer layer comparable to the dura mater. At 6 to 7 postmenstrual weeks, the choroid originates from the inner layer, and the outer layer develops into the sclera. The anterior chamber of the eye comes from the mesenchyme that overlies the lens. At 8 postmenstrual weeks, the cornea differentiates from the external layer of the anterior chamber. The eyelids are mesodermal folds lined with ectoderm that meet in front of the cornea by the eighth week.10,11
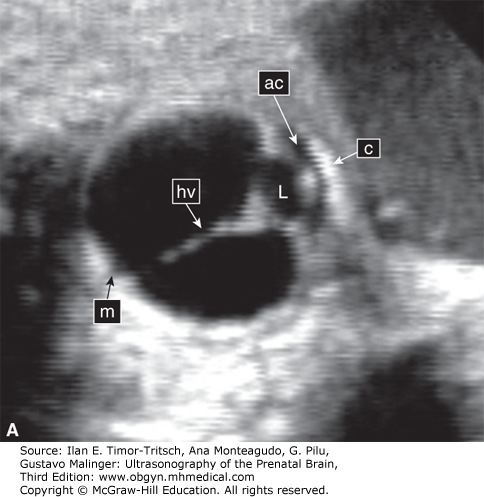
The hyaloid artery originates from the ophthalmic artery. It runs through the center of the eye and terminates at the posterior surface of the lens (Figure 14–2).10,11,12 Its primary function is to nourish the developing lens. There is a normal process of regression of the hyaloid vessels toward the end of pregnancy.10,11,12,13 A delay in the process of regression is associated with fetal abnormalities mainly of the central nervous system (CNS).13 The flow in the hyaloid artery can be seen using color Doppler sonography. Figure 14–2B depicts hyaloid arterial flow at 16 weeks.
Ocular growth during fetal life was studied in products of spontaneous and induced abortions.14,15 The development of the diameter and circumference of the eyeball showed an almost linear increase during gestation. The horizontal diameter of the eyeball was longer than the sagittal, and the vertical diameter was the shortest one. The average diameters of the eye in male fetuses were longer than in female fetuses.14 At birth, the various dimensions of the eyeball were approximately one-third of the adult size.
A detailed ultrasonic examination of the fetal eye was first reported by Birnholz16 and de Elejalde and Elejalde.17 The eyes were analyzed in both axial and coronal planes. In the axial plane, scanning is done from the top of the skull across the fetal face. In the coronal plane, the scanning focus was moved from the tip of the nose to the posterior aspect of the eye.16,17 In both studies, the eyelids, lens, cornea, sclera, irises, hyaloid artery, retina, and optic nerve were depicted. However, such a detailed examination is not always possible, and sonologists are advised to refer to these studies in order to better understand the ultrasonic features of the different parts of the fetal eye.16,17 For practical purposes, most sonologists examine qualitatively the size and location of the orbits, eyelids, hyaloid artery, and lens (Figures 14–2, 14–3, 14–4, 14–5, 14–6, and 14–7). Whenever an ocular malformation is suspected and the fetus is in the vertex presentation, the use of transvaginal sonography (TVS) may in some cases provide better visualization of the different eye structures. The use of high-resolution abdominal probes enables good visualization of these structures.
Nomograms have been constructed providing data on fetal axial eye length, interocular distance, binocular distance, lens diameter, and a timetable for hyaloid artery regression and flow cessation.18,19,20,21,22,23,24 Use of these nomograms may be helpful in the detection of fetal hypo- or hypertelorism, as well as other fetal eye and face malformations (Tables 14–1 and 14–2). (See also Chapter 3.)
| Age (postmenstrual weeks) | Binocular Distance (mm) | Interocular Distance (mm) | Ocular Diameter (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 50th | 95th | 5th | 50th | 95th | 5th | 50th | 95th | |
| 11 | 5 | 13 | 20 | — | — | — | — | — | — |
| 12 | 8 | 15 | 23 | 4 | 9 | 13 | 1 | 3 | 6 |
| 13 | 10 | 18 | 25 | 5 | 9 | 14 | 2 | 4 | 7 |
| 14 | 13 | 20 | 28 | 5 | 10 | 14 | 3 | 5 | 8 |
| 15 | 15 | 22 | 30 | 6 | 10 | 14 | 4 | 6 | 9 |
| 16 | 17 | 25 | 32 | 6 | 10 | 15 | 5 | 7 | 9 |
| 17 | 19 | 27 | 34 | 6 | 11 | 15 | 5 | 8 | 10 |
| 18 | 22 | 29 | 37 | 7 | 11 | 16 | 6 | 9 | 11 |
| 19 | 24 | 31 | 39 | 7 | 12 | 16 | 7 | 9 | 12 |
| 20 | 26 | 33 | 41 | 8 | 12 | 17 | 8 | 10 | 13 |
| 21 | 28 | 35 | 43 | 8 | 13 | 17 | 8 | 11 | 13 |
| 22 | 30 | 37 | 44 | 9 | 13 | 18 | 9 | 12 | 14 |
| 23 | 31 | 39 | 46 | 9 | 14 | 18 | 10 | 12 | 15 |
| 24 | 33 | 41 | 48 | 10 | 14 | 19 | 10 | 13 | 15 |
| 25 | 35 | 42 | 50 | 10 | 15 | 19 | 11 | 13 | 16 |
| 26 | 36 | 44 | 51 | 11 | 15 | 20 | 12 | 14 | 16 |
| 27 | 38 | 45 | 53 | 11 | 16 | 20 | 12 | 14 | 17 |
| 28 | 39 | 47 | 54 | 12 | 16 | 21 | 13 | 15 | 17 |
| 29 | 41 | 48 | 56 | 12 | 17 | 21 | 13 | 15 | 18 |
| 30 | 42 | 50 | 57 | 13 | 17 | 22 | 14 | 16 | 18 |
| 31 | 43 | 51 | 58 | 13 | 18 | 22 | 14 | 16 | 19 |
| 32 | 45 | 52 | 60 | 14 | 18 | 23 | 14 | 17 | 19 |
| 33 | 46 | 53 | 61 | 14 | 19 | 23 | 15 | 17 | 19 |
| 34 | 47 | 54 | 62 | 15 | 19 | 24 | 15 | 17 | 20 |
| 35 | 48 | 55 | 63 | 15 | 20 | 24 | 15 | 18 | 20 |
| 36 | 49 | 56 | 64 | 16 | 20 | 25 | 16 | 18 | 20 |
| 37 | 50 | 57 | 65 | 16 | 21 | 25 | 16 | 18 | 21 |
| 38 | 50 | 58 | 65 | 17 | 21 | 26 | 16 | 18 | 21 |
| 39 | 51 | 59 | 66 | 17 | 22 | 26 | 16 | 19 | 21 |
| 40 | 52 | 59 | 67 | 18 | 22 | 26 | 16 | 19 | 21 |
| Centiles | |||||||
|---|---|---|---|---|---|---|---|
| GA (postmenstrual weeks) | Mean | 95% Cl | 10 | 25 | 50 | 75 | 90 |
| 14 | 2.5 | 2.3–2.7 | 2.1 | 2.4 | 2.5 | 2.7 | 2.9 |
| 15 | 2.9 | 2.9–3.0 | 2.7 | 2.8 | 2.9 | 3.1 | 3.2 |
| 16 | 2.9 | 2.8–3.0 | 2.7 | 2.8 | 2.9 | 3.1 | 3.2 |
| 17–18 | 3.3 | 3.0–3.6 | 2.8 | 2.9 | 3 | 3.3 | 5 |
| 19–20 | 4.1 | 4.0–4.3 | 3.6 | 4 | 4 | 4.3 | 5 |
| 21 | 4.4 | 4.1–4.6 | 3.7 | 3.9 | 4 | 5 | 5 |
| 22 | 4.4 | 4.2–4.7 | 3.9 | 4 | 4.3 | 5 | 5 |
| 23 | 4.6 | 4.3–4.8 | 3.8 | 4 | 5 | 5 | 5 |
| 24 | 4.6 | 4.4–4.8 | 4 | 4.3 | 4.6 | 5 | 5 |
| 25 | 4.8 | 4.6–5.0 | 4.2 | 4.6 | 5 | 5.1 | 5.2 |
| 26 | 5 | 4.8–5.2 | 4.4 | 4.8 | 5.1 | 5.2 | 5.5 |
| 27 | 5 | 4.8–5.2 | 4.4 | 4.8 | 5.1 | 5.2 | 5.5 |
| 28 | 5.1 | 5.0.5.2 | 4.5 | 5 | 5.2 | 5.2 | 5.5 |
| 29 | 5.3 | 5.1–5.5 | 4.6 | 5.2 | 5.2 | 5.5 | 5.9 |
| 30–31 | 5.3 | 5.2–5.5 | 4.8 | 5.1 | 5.5 | 5.5 | 5.7 |
| 32–33 | 5.6 | 5.4–5.8 | 4.8 | 5.2 | 5.5 | 5.9 | 6.2 |
| 34–36 | 5.8 | 5.6–6.0 | 5.4 | 5.5 | 5.7 | 6 | 6.5 |
Although the nomograms are useful and important, we do not routinely measure the ocular biometry in low-risk pregnancies, but instead use a qualitative approach comparing the two orbits and eyes in the same plane. When a facial anomaly is suspected, as well as in patients with a familiar history of malformations involving the eyes, we recommend a more extensive investigation that includes measurements and comparison with nomograms.
The normal lens appears on the coronal ultrasonic facial view as a smooth, hyperechogenic circular line with a hypoechogenic content (Figures 14–4 and 14–5). In the axial view of the fetal head and orbits, the lenses are visualized as a pair of small dotted echoes originating from their near and far margins (Figure 14–2).
Cataracts are opacities of the lens that cause visual impairment. The mechanism of cataract formation is denaturation of lens protein and formation of an opaque insoluble precipitate that causes loss of lens translucency.25 This may be unilateral or bilateral. It has been estimated that this anomaly accounts for ~10% of the cases of blindness in preschool children.25,26 Approximately one-third of cataract cases are idiopathic, and many of these are familial. Both autosomal dominant and autosomal recessive inheritance have been reported.26,27 Other possible etiologies are intrauterine infections, chromosomal disorders, and systemic syndromes.25,26,27,28
Cataracts may produce different US imaging patterns. Thick, irregular, or crenate hyperechogenic borders, clusters of hypoechogenic material, or homogeneous opacities may be observed (Figures 14–8, 14–9, 14–10, 14–11, and 14–12).29,30,31,32,33,34,35,36,37 In some cases of cataracts, we failed to demonstrate the hyaloid artery. It is possible that the opacity of the lens interfered with the visualization of this vessel. It can also be speculated that there is an association between congenital cataract and pathologies of the hyaloid artery.38
Figure 14–9.
Homogeneous hyperechogenicity of the right lens and clusters of hypoechogenic material in the left eye (cataract). (From Zimmer et al, 1993,26 with permission.)

Figure 14–11.
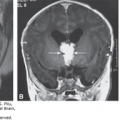
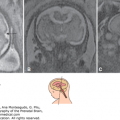
Atrophy of the eyelids (A) Eyelids (EL). The upper and lower eyelids (arrow) were small and open throughout the ultrasound (US) examination. Partial atrophy was therefore suggested. (B) The aborted fetus with Neu-Laxova syndrome. There is partial atrophy of the eyelids, as well as cataract.


It should be noted that demonstration of apparently normal lenses on US does not exclude the presence of cataract. Additionally, the transparency of the lens on clinical examination is due to the free passage of light, whereas sonolucency refers to free passage of US waves through the lens. We have failed to diagnose some cases of mild cataract.31 Therefore, it is understandable and can be summarized that at present the threshold for the ultrasonic identification of cataract is still uncertain, and mild to moderate or even severe cases might not be sufficiently abnormal for sonographic detection and recognition.32,33 As for the earliest possible detection of congenital cataracts, Monteagudo et al33 reported an early diagnosis using transvaginal scan at 14 weeks of gestation.
Because the normal eyelid and its opening and closure can be detected, it is expected that a thorough, targeted examination of the eyelids can potentially detect abnormalities. Figure 14–11A depicts a fetus with fixed, partially open eyelids detected during ultrasonography. Examination of the stillborn fetus confirmed this finding. The detected syndrome was the Neu-Laxova syndrome (Figure 14–11B); similar findings have been reported by Shapiro and associates.30