Radiologists play a key role in identifying, characterizing, sampling, and, in some cases, treating abnormalities of the spinal bone marrow. This article discusses the composition and dynamic nature of the bone marrow, and how these changes directly correlate with the imaging appearance. Examples of entities that are diffuse, infiltrative, solitary, and multifocal are shown and diagnostic dilemmas and mimics are described The radiologist must be aware of the expected appearance and variability of the bone marrow in order to provide timely, accurate diagnoses.
Key points
- •
Bone marrow appearance on MR imaging reflects its composition. The dynamic nature of marrow in health and disease, as well as individual variability makes characterization challenging.
- •
T1, T2, and fat-saturated T2 images are required to comprehensively evaluate marrow. Contrast administration is optional.
- •
Patients with hematologic malignancies may have persistent or new T1 hypointensity throughout the spinal column due to diseases, anemia, or treatment.
- •
Mimics of osseous metastatic disease include benign enostosis, aggressive hemangioma, and some infections, such as coccidioidomycosis.
Introduction
The bone marrow is one of the largest organs in the body. It is composed of hematopoietic cells, adipocytes, and supportive stromal cells surrounded by vascular sinuses and trabecular bone enclosed within a cortical shell. Marrow composition changes with physiologic needs related to age, gender, nutritional status, illness, comorbid conditions, and other factors. Marrow adipose tissue plays a role in bone and systemic health through its interactions with the surrounding osseous structure and has been discovered to contribute to metabolic disorders such as osteoporosis and diabetes mellitus. , The dynamic nature of the bone marrow makes imaging evaluation challenging, given the substantial variability between individuals and in a given individual throughout life, with physiologic changes during development, stress, disease, and treatment.
The marrow compartment may be affected by a number of benign and malignant processes, including hematologic malignancy, primary tumors, and metastases. Metabolic conditions, such as osteoporosis, as well as infection, inflammatory processes, and trauma, also influence marrow composition and imaging characteristics. Given the volume of vertebral marrow and its central location in the majority of imaging studies, all radiologists should have an understanding of the composition, variability, and normal and abnormal imaging characteristics to provide an accurate assessment.
Vertebral bone marrow variability in heath and disease
Marrow Composition and Imaging Appearance
The basic marrow components, fat, water, and protein, vary in volume with normal growth and in response to different stimuli. Hence, their proportions are the main determinants of spinal marrow MR signal characteristics. The cellular composition of hematopoietic “red marrow” is 60% hematopoietic cells and 40% fat cells. With age, the marrow converts to fatty “yellow marrow,” with marked increase in fat (95%) and decrease in hematopoietic cells (5%).
The spectrum of vertebral bone marrow signal variations ranges from benign, as with hyperplastic red marrow, to malignant, as in the case of hematologic malignancy and metastatic disease. The pathologic process can be diffuse throughout the marrow or present with localized mass. Normally, bone marrow is diffusely hematopoietic from childhood through young adulthood ( Fig. 1 ). T1 is the MR sequence of choice to evaluate vertebral bone marrow-related changes. Red marrow is usually slightly hyperintense to normal intervertebral disc. As aging continues, vertebral bone marrow will convert to primarily fatty marrow that is substantially more T1 hyperintense than disc and muscle. Bone marrow signal that is hypointense to adjacent disc or muscle using 1.5 T field strength is abnormal with accuracy of 94% and 98%. With 3 Tesla (3T) imaging, comparing marrow T1 signal to muscle is more sensitive for abnormal intensity. With again and conversion, 3 patterns are usually observed: fatty replacement in a band-like configuration along the endplates, larger confluent areas of marrow replacement, or small focal areas of marrow replacement. In addition to changes with aging, bone marrow composition, and thus imaging appearance, changes in reaction to stressors. Known environmental factors that cause red marrow hyperplasia include anemia, changes in diet, chemotherapy, chronic hypoxia that can occur with smoking, and treatment with granulocyte colony-stimulating factor.

T1 and STIR or fat-saturated T2 images are required for marrow evaluation. Contrast-enhanced imaging is optional. Dixon is a reliable MR imaging technique that can be used for problem-solving in the assessment of bone marrow lesions. Dixon techniques rely on the difference in resonance frequency between fat and water, and in a single acquisition, fat-only, water-only, in-phase, and out-of-phase images are acquired. This allows for quantification of fat within a lesion, improving the ability to differentiate marrow infiltrating and non-infiltrating processes such as focal nodular marrow hyperplasia. Dixon can be used with gradient echo and spin echo techniques, and both 2 dimensional and 3 dimensional imaging. Another advantage is its rapid acquisition time, especially when using traditional 2 point Dixon gradient echo sequences. This robust fat suppression method can also be used with intravenous contrast agents.
Infiltrative processes: hematologic malignancy
Hematologic malignancies, including multiple myeloma (MM), lymphoma, and leukemia, generally affect the entire marrow compartment. In all diffusely marrow infiltrative diseases, MR imaging shows homogenous or heterogeneous low T1 signal, with enhancement on post-contrast T1 images and commensurate elevated T2 signal.
Focal and diffuse marrow compartment processes are often associated with vertebral compression fractures. Infrequently, complete collapse of the vertebral body, or vertebral plana, occurs in leukemia, and more rarely in myeloma and lymphoma. Bone infarcts and bone necrosis may be seen in leukemia.
Multiple Myeloma
MM is a hematologic malignancy characterized by clonal expansion of malignant plasma cells within the marrow. This is the second most common hematologic malignancy, comprising 10% of these cancers. The incidence of MM increases with age, with a median age at diagnosis of 69 years. Prevalence is rising as the population ages. Overall, 30% of patients are aged over 75 years. The 5 year relative survival rate is approximately 40% but survival is increasing with discovery of new treatments.
Osteolytic lesions develop in the majority of patients with MM. Over 80% of newly diagnosed patients with MM will develop detectable bone disease. At the cellular level, there is a tumor-cell-driven decoupling of osteoclast and osteoblast regulation, similar to that in osteoporosis. Overall, 50% of patients with myeloma will experience skeletal-related events (SREs), such as pathologic fracture and spinal cord compression, which increase the risk of mortality by 20% to 40%.
Imaging for patients with MM serves to distinguish between asymptomatic and symptomatic disease, depending upon visible bone disease. After diagnosis and therapy, imaging is utilized to assess treatment efficacy and disease stability. In all phases of disease, radiologists can add tremendous value by calling attention to lesions with elevated risk of pathologic fracture, which may require surgical stabilization regardless of treatment efficacy. Cement augmentation is frequently used for stabilization and pain control, due to its effectiveness and safety. ,
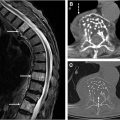
Early diagnosis and effective treatment are critical in improving outcomes for patients with MM. Studies have found that to detect lesions with conventional radiographs, 30% to 50% of the bone needs to be destroyed. Unlike other malignancies that metastasize to bone, the osteolytic bone lesions in MM exhibit no new bone formation. For this reason, where available, computed tomography (CT), MR imaging, and/or FDG-PET are used to increase sensitivity. CT is sensitive for the detection of small lytic lesions ( Fig. 2 ) and allows for biopsy planning and assessment of fracture risk. Lytic lesions do not resolve even after successful therapy, so CT is less useful in the posttreatment setting. Low-dose whole-body CT is the modality of choice for initial assessment.

Lesions are well circumscribed with a narrow zone of transition. There may be cortical destruction and soft tissue extension of disease. These patients do not need and often cannot safely receive, intravenous contrast due to their decreased renal function secondary to the disease.
Current consensus for myeloma diagnosis requires 10% or more clonal bone marrow plasma cells or a biopsy-proven plasmacytoma plus evidence of one or more multiple myeloma-defining events: CRAB hypercalcemia, renal failure, anemia, or lytic bone lesions attributable to the plasma cell disorder, bone marrow clonal plasmacytosis 60% or greater, serum involved/uninvolved free light chain (FLC) ratio 100 or greater (provided involved FLC is ≥100 mg/L and urine monoclonal protein is ≥200 mg/24 h), or greater than 1 focal lesion on MR imaging.
MR patterns have been described by the International Myeloma Working Group: diffuse, diffuse and focal, focal, salt and pepper, and normal , ( Fig. 3 ). This subjective assessment reflects the tumor burden, impacting treatment and prognosis, with focal and diffuse patterns having a worse prognosis. On MR imaging, lesions have low signal on T1, high signal on T2, STIR, and fat-saturated T2 images and enhancement. In treated patients, red marrow reconversion, also with T1 hypointensity, is common due to anemia, chemotherapy, and marrow stimulating factors. Diffusion and dynamic contrast-enhanced perfusion imaging is also used for disease diagnosis, staging, and to evaluate treatment response.

Approximately 40% of individuals with myeloma will develop a pathologic fracture in the year after diagnosis, and 60% later in the course of the disease. MR imaging features that aid in differentiating pathologic from osteoporotic fractures, common in these patients, include associated enhancing paraspinal or epidural soft tissue, pedicle or posterior element involvement, expansion or bowing of the posterior cortex, and lesions at additional levels.
Plasmacytoma
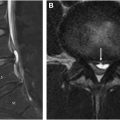
Plasmacytoma is a localized proliferation of neoplastic monoclonal plasma cells without evidence of systemic disease. Solitary bone plasmacytoma is rare, and these often are the early manifestation of MM. The purpose of imaging in plasmacytoma is to evaluate the tumor for treatment planning, including biopsy for histologic confirmation, and evaluating the rest of the body for additional lesions. After treatment, follow-up imaging is performed to evaluate the efficacy of treatment and again, surveil for additional sites of disease, that is, evolution to myeloma.
Diagnosis depends upon biopsy showing clonal plasma cells, normal bone marrow biopsy, otherwise normal imaging of the spine and pelvis, and absence of end-organ damage seen in myeloma (renal insufficiency, anemia, hypercalcemia).
On CT, plasmacytoma is primarily lytic with expansion, and thinning of the cortex. There are often enlarged vertical trabecular struts, which thicken to provide support to the vertebral body as it loses its normal internal supportive structure with expansion of the neoplastic cells. On MR, these lesions are T1 hypointense, T2 hyperintense, and enhancement ( Fig. 4 ).

Plasmacytoma may be treated with chemotherapy, bisphosphonates, radiation, or surgery. Of note to radiologists, nearly half of patients treated with radiotherapy demonstrate reossification of the affected body. These patients were also shown to have a better prognosis, with less possibility of progression to myeloma.
Lymphoma and Leukemia
Malignant involvement of the vertebral column can be diffuse, focal, or a mixture of both patterns. Lymphoma and leukemia tend to diffusely infiltrate the spinal column.
Early bone marrow changes of hematologic malignancy can be difficult to identify, especially in young patients with red marrow and when disease is unexpected as imaging is performed for another purpose. Because normal red marrow can have an unusual appearance at MR imaging, particularly on fluid-sensitive or post-contrast images, the radiologist must be able to recognize normal red marrow, physiologic red marrow reconversion, and differentiate these from bone marrow disease.
Despite the known patterns of red marrow conversion and reconversion, isolated islands of residual or reconverted red bone marrow in either typical or atypical locations can be confused with infiltrative bone marrow disease. In this case, in and out of phase or Dixon imaging can help with problem-solving. As previously described, red marrow is composed of approximately 40% fat. When it is imaged at an echo time when fat and water are out of phase, signal from intravoxel fat and water will drop relative to an in-phase image. Yellow marrow, with its higher fat content, will experience little signal drop on out-of-phase images. Pathologic conditions that replace rather than infiltrate normal marrow also will not show signal drop on out-of-phase images as they do not contain a significant volume of fat.
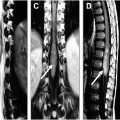
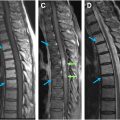
Lymphoma is a heterogeneous group of malignant B-cell and T-cell neoplasms. The presence of bone marrow involvement is a key diagnostic consideration in management decisions. The most common lymphoma affecting the spinal column is non-Hodgkin’s lymphoma. The bone marrow is involved in up 45% of cases. There may be diffuse or multifocal marrow involvement and permeative destruction. On CT, one may see lytic or permeative bone destruction and paraspinal or epidural mass. On MR imaging, lesions are T1 hypointense, variable on T2, and with diffuse enhancement ( Fig. 5 ).

Leukemia infiltrates bone marrow with neoplastic monoclonal cells. As in other infiltrative processes, there is decreased signal intensity on T1-weighted images, increased signal intensity on T2-weighted fat-saturated or STIR images, and usually diffuse enhancement ( Fig. 6 ). The most commonly reported radiographic finding of leukemic bone marrow infiltration is osteopenia, but it also presents lytic or permeative bone destruction, soft tissue mass, and pathologic fracture.

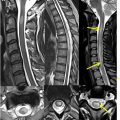
Both bone marrow infarcts and bone marrow necrosis can occur in patients with leukemia. Bone marrow necrosis is loss of the myeloid tissue and marrow fat without loss of trabecula. It may be caused by vascular occlusion by deformed red blood cells, tumor emboli, anemia, infection, radiation or chemotherapy, or release of tumor necrosis factor. These cases show markedly abnormal marrow signal, but the risk of pathologic fracture is less than in the case of tumor infiltration. Marrow necrosis can also rarely be seen in lymphoma, sickle cell anemia, and infection. On MR imaging, these are geographic lesions with layered T2 hypointensity and hyperintensity and enhancement ( Fig. 7 ).

Osseous metastatic disease
Osseous metastatic disease is by far the most common tumor of the spine, and imaging is central to detection and diagnosis. All radiologists will encounter subtle and advanced cases of metastatic disease in the emergency, outpatient, and inpatient settings. Spinal metastases are increasingly common: the population is aging, treatments are improving, and patients with cancer are living longer with their disease. Despite advances in the understanding and treatment of cancer, spinal column metastases remain difficult yet necessary to treat due to the implications of pathologic spinal column failure. Pathologic fracture or epidural extension of disease produces substantial morbidity and mortality.
MR imaging is the most sensitive technique for detection and characterization of osseous metastases: evaluation with T1-weighted, T2-weighted, and STIR sequences is more than 98% sensitive and specific. Marrow infiltration is detected well before trabecular and cortical destruction are seen on CT or radiographs. Metastases are T1 hypointense and can become isointense to normal marrow on post-contrast images, making fat suppression useful for detection on contrast-enhanced images. Contrast-enhanced Dixon images, with fat water separation, can provide even greater conspicuity of metastatic lesions ( Fig. 8 ). This technique provides increased background suppression on opposed phase images, which cancels signal from marrow water and fat, and fat-only images, in which infiltrative lesions appear dark compared with normal hyperintense marrow fat. Sclerotic metastases are hypointense on T1-weighted and T2-weighted images and may have little or no enhancement after contrast administration.