Recommended breast cancer proton therapy indications
Indications
- 1)
Accelerated partial breast irradiation
- ■
The safety and efficacy of proton partial breast irradiation (PBI) is established for early-stage breast cancer. Proton PBI provides a more homogeneous dose distribution and reduction in exposure to the normal breast, heart, and lung compared with photon and brachytherapy PBI techniques and has been associated with excellent local control and reduced toxicity. [CR] We are investigating a 10-fraction hypofractionated proton regimen, which may be more cost-effective and also improve the therapeutic ratio.
- ■
Inclusion criteria
- i.
The patient must have stage 0, I, or II breast cancer. If stage II, the tumor size must be 3 cm or less.
- ii.
On histological examination, the tumor must be ductal carcinoma in situ (DCIS) or invasive adenocarcinoma of the breast.
- iii.
Surgical treatment of the breast must have been lumpectomy. The margins of the resected specimen must be histologically free of tumor (DCIS and invasive). Reexcision of surgical margins is permitted.
- iv.
Gross disease must be unifocal with pathologic (invasive and/or DCIS) tumor size 3 cm or less. (Patients with microscopic multifocality are eligible as long as total pathologic tumor size is ≤3 cm.)
- v.
The target lumpectomy cavity must be clearly delineated, and the target lumpectomy cavity/whole breast reference volume must be less than or equal to 30% based on the postoperative/preenrollment computed tomography (CT) scan.
- i.
- ■
Exclusion criteria:
- i.
Men are not eligible.
- ii.
T2 (>3 cm), T3, stage III, or stage IV breast cancer.
- iii.
More than three histologically positive axillary nodes.
- iv.
Axillary nodes with definite evidence of microscopic or macroscopic extracapsular extension.
- v.
Palpable or radiographically suspicious ipsilateral or contralateral axillary, supraclavicular, infraclavicular, or internal mammary nodes at time of enrollment unless there is histologic confirmation that these nodes are negative for tumor.
- vi.
Suspicious microcalcifications or densities (in the ipsilateral or contralateral breast as documented on mammogram or breast ultrasound) unless biopsied and found to be benign.
- vii.
Nonepithelial breast malignancies such as sarcoma or lymphoma.
- viii.
Proven multicentric carcinoma (invasive cancer or DCIS) in more than one quadrant or separated by 4 or more centimeters.
- ix.
Paget disease of the nipple.
- x.
Surgical margins that cannot be microscopically assessed or are positive at pathologic evaluation. (If surgical margins are rendered free of disease by re-excision, the patient is eligible.)
- xi.
Clear delineation of the extent of the target lumpectomy cavity not possible.
- xii.
Treatment plan that includes regional nodal irradiation.
- xiii.
Prior radiation to the index breast.
- xiv.
Documented diagnosis of collagen vascular disease, specifically dermatomyositis with a creatine phosphokinase level above normal or with an active skin rash, systemic lupus erythematosus, or scleroderma.
- xv.
Pregnancy or lactation at enrollment.
- xvi.
Women of reproductive potential must agree to use an effective nonhormonal method of contraception during therapy.
- i.
- ■
- 2)
Left or right-sided early or locoregionally advanced breast cancer requiring breast or chest wall plus regional nodal irradiation (i.e., lymph node-positive disease, advanced T stage, and/or medial tumor location)
- ■
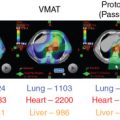
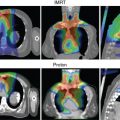
Adjuvant radiotherapy improves survival in breast cancer patients, suggesting that persistence of locoregional tumor is associated with an increased risk of developing metastases and death. Results of modern randomized controlled clinical trials highlight the importance of regional nodal irradiation in reducing distant events in this population. [CR] , [CR] Targeting of the regional lymphatics results in lung and heart doses associated with increased major cardiac events, cardiac deaths, lung cancer, and lung cancer deaths in a patient population where advances in systemic therapy and other multidisciplinary care has resulted in decreasing breast cancer-specific mortality. Proton radiotherapy improves coverage of the regional lymphatics while substantially reducing mean lung and mean heart doses (MHDs) to levels significantly correlated with reduced cardiac events, lung cancer, and symptomatic pneumonitis. [CR] We are currently enrolling patients in the national RADCOMP trial (NCT02603341) that is randomizing patients who receive regional nodal irradiation between proton and photon radiation.
- ■
Inclusion criteria
- i.
Age 18 years or older.
- ii.
Histologic confirmation of breast cancer resected by lumpectomy or mastectomy with or without immediate reconstruction and whole breast/chest wall and regional nodal irradiation planned with or without a boost to the lumpectomy cavity/chest wall.
- iii.
The axilla must be staged by sentinel node biopsy alone, sentinel node biopsy followed by axillary node dissection, or axillary lymph node dissection alone.
- iv.
pStage T1-T4N0-N3M0 or ypStage T0-4N0-N3M0.
- v.
Indications for regional nodal irradiation per treating physician (lymph node–positive disease, T3 to T4, medial tumor location).
- vi.
Breast implants and expanders allowed.
- i.
- ■
Exclusion criteria
- i.
Medical contraindication to receipt of radiotherapy.
- ii.
Severe, active comorbid systemic illnesses or other severe concurrent disease that, in the judgment of the investigator, would make the patient inappropriate for entry into this study or interfere significantly with the proper assessment of safety and toxicity of the prescribed regimens.
- iii.
Active systemic lupus or scleroderma.
- iv.
Pregnancy or women of childbearing potential who are sexually active and not willing/able to use medically acceptable forms of contraception.
- i.
- ■
Scientific evidence
- 1.
Ares C, Khan S, Macartain AM, et al. Postoperative proton radiotherapy for localized and locoregional breast cancer: potential for clinically relevant improvements? Int J Radiat Oncol Biol Phys. 2010;76:685-697.
- 2.
Bradley JA, Dagan R, Ho MW, et al. Initial report of a prospective dosimetric and clinical feasibility trial demonstrates the potential of protons to increase the therapeutic ratio in breast cancer compared with photons. Int J Radiol Oncol Biol Phys. 2016;95(1): 411-421.
- 3.
Bush DA, Do S, Lum S, et al. Partial breast radiation therapy with proton beam: 5-year results with cosmetic outcomes. Int J Radiat Oncol Biol Phys. 2014;90(3):501-505.
- 4.
Bush DA, Slater JD, Garberoglio C, Do S, Lum S, Slater JM. Partial breast irradiation delivered with proton beam: results of a phase II trial. Clin Breast Cancer. 2011;11(4): 241-245.
- 5.
Chang JH, Lee NK, Kim JY, et al. Phase II trial of proton beam accelerated partial breast irradiation in breast cancer. Radiother Oncol. 2013;108(2):209-214.
- 6.
Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087-2106.
- 7.
Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6(8):557-565.
- 8.
Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998.
- 9.
Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804): 1707-1716.
- 10.
Depauw N, Batin E, Daartz J, et al. A novel approach to postmastectomy radiation therapy using scanned proton beams. Int J Radiat Oncol Biol Phys. 2015;91(2): 427-434.
- 11.
Doyen J, Falk AT, Floquet V, et al. Proton beams in cancer treatments: clinical outcomes and dosimetric comparisons with photon therapy. Cancer Treat Rev. 2016;43: 104-112.
- 12.
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-2135.
- 13.
Galland-Girodet S, Pashtan I, MacDonald SM, et al. Long-term cosmetic outcomes and toxicities of proton beam therapy compared with photon-based 3-dimensional conformal accelerated partial-breast irradiation: a phase 1 trial. Int J Radiat Oncol Biol Phys. 2014; 90(3):493-500.
- 14.
Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013; 14(11):1086-1094.
- 15.
Johansson J, Isacsson U, Lindman H, Montelius A, Glimelius B. Node-positive left-sided breast cancer patients after breast-conserving surgery: potential outcomes of radiotherapy modalities and techniques. Radiother Oncol. 2002;65(2):89-98.
- 16.
Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet . 1999;353(9165):1641-1648.
- 17.
MacDonald SM, Patel SA, Hickey S, et al. Proton therapy for breast cancer after mastectomy: early outcomes of a prospective clinical trial. Int J Radiat Oncol Biol Phys . 2013;86(3):484-490.
- 18.
MacDonald SM, Jimenez R, Paetzold P, et al. Proton radiotherapy for chest wall and regional lymphatic radiation; dose comparisons and treatment delivery. Radiat Oncol . 2013;8:71.
- 19.
Mast ME, Vredeveld EJ, Credoe HM, et al. Whole breast proton irradiation for maximal reduction of heart dose in breast cancer patients. Breast Cancer Res Treat . 2014;148(1):33-39.
- 20.
McGee LA, Iftekaruddin Z, Chang JHC, et al. Postmastectomy chest wall reirradiation with proton therapy for breast cancer. Int J Radiat Oncol Biol Phys . 2017;99(2):E34-E35.
- 21.
Moon SH, Shin KH, Kim TH, et al. Dosimetric comparison of four different external beam partial breast irradiation techniques: three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, helical tomotherapy, and proton beam therapy. Radiother Oncol . 2009;90(1):66-73.
- 22.
Plastaras JP, Berman AT, Freedman GM. Special cases for proton beam radiotherapy: re-irradiation, lymphoma, and breast cancer. Semin Oncol . 2014;41(6):807-819.
- 23.
Olivotto IA, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol . 2013;31(32):4038-4045.
- 24.
Ovalle V, Strom EA, Shaitelman S, et al. Proton partial breast irradiation: detailed description of acute clinico-radiologic effects. Cancers (Basel) . 2018;10(4).
- 25.
Ovalle V, Strom EA, Godby J, et al. Proton partial-breast irradiation for early-stage cancer: is it really so costly? Int J Radiat Oncol Biol Phys . 2016;95(1):49-51.
- 26
Patel SA, Tan T, Chin Y, et al. Assessment of cardiac function following proton radiation in a cohort of post mastectomy patients with locally advanced breast cancer. Int J Radiat Oncol Biol Phys . 2015;93(3 Suppl):e52.
- 27.
Poortsman PM, Collette S, Kirkove C, et al. Internal mammary and mediastinal supraclavicular irradiation in breast cancer. N Engl J Med . 2015;373(4):317-327.
- 28.
Presley CJ, Soulos PR, Herrin J, et al. Patterns of use and short-term complications of breast brachytherapy in the national Medicare population from 2008–2009. J Clin Oncol . 2012;30(35):4302-4307.
- 29.
Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med . 1997;337(14):956-962.
- 30.
Shah C, Badiyan S, Berry S, et al. Cardiac dose sparing and avoidance techniques in breast cancer radiotherapy. Radiother Oncol . 2014;112(1):9-16.
- 31.
Stick LB, Yu Jen, Maraldo MV, et al. Joint estimation of cardiac toxicity and recurrence risks after comprehensive nodal photon versus proton therapy for breast cancer. Int J Radiat Oncol Biol Phys . 2017;97(4):754-761.
- 32.
Strom EA, Amos R, Shaitelman SF, et al. Proton partial breast irradiation in the supine position: treatment description and reproducibility of a multibeam technique. Pract Radiat Oncol . 2015;5(4):e283-e290.
- 33.
Strom EA, Ovalle V. Initial clinical experience using protons for accelerated partial-breast irradiation: longer-term results. Int J Radiat Oncol Biol Phys . 2014;90(3): 506-508 .
- 34.
Taylor CW, Wang Z, Macaulay E, et al. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys . 2015;93(4):845-853.
- 35.
Verma V, Shah C, Mehta M. Clinical outcomes and toxicity of proton radiotherapy for breast cancer. Clin Breast Cancer . 2016;16(3):145-154.
- 36.
Verma V, Iftekaruddin Z, Badar N, et al. Proton beam radiotherapy as part of comprehensive nodal irradiation for locally advanced breast cancer. Radiother Oncol . 2017; 123(2):294-298.
- 37.
Wang X, Zhang X, Li X, et al. Accelerated partial-breast irradiation using intensity-modulated proton radiotherapy: do uncertainties outweigh potential benefits? Br J Radiol . 2013;86(1029):20130176.
- 38.
Wang X, Amos RA, Zhang X, et al. External-beam accelerated partial breast irradiation using multiple proton beam configurations. Int J Radiat Oncol Biol Phys . 2011;80(5): 1464-1472.
- 39.
Whelan TJ, Olivotto I, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Eng J Med . 2015;373(4):307-316.
- 40.
Xu N, Ho MW, Li Z, Morris CG, Mendenhall NP. Can proton therapy improve the therapeutic ratio in breast cancer patients at risk for nodal disease? Am J Clin Oncol . 2014;37:568-574.
Recommended central nervous system proton therapy indications
Indications
- 1.
Adult craniospinal radiotherapy : primary central nervous system (CNS) tumors
- a.
Decrease acute toxicity
- i.
Reduce weight loss, hydration issues, nausea, vomiting [CR]
- ii.
Minimize treatment breaks that affect disease control [CR]
- iii.
Improve chemotherapy tolerance
- i.
- b.
Reduce late toxicities
- c.
Improve quality of life and symptom burden
- a.
- 2.
Adult low-grade glioma and anaplastic oligodendroglioma in whom long-term survival is expected [CR] , [CR]
- a.
Decrease neuroendocrine and auditory toxicity
- b.
Decrease neurocognitive deficits [CR]
- c.
Improve quality of life and symptom burden
- a.
- 3.
Selected meningiomas and Sellar tumors: large tumors, young patients, other comorbidities (neurofibromatosis, Li-Fraumeni, etc.)
- a.
Evidence of better survival in aggressive meningioma with higher dose possible with proton radiation therapy [CR]
- b.
Long-term survival expectation
- c.
Decrease neuroendocrine, auditory toxicity
- d.
Decrease neurocognitive deficits
- e.
Improve quality of life and symptom burden
- a.
- 4.
Recurrent, previously irradiated tumors in the brain, thorax, or abdomen: proton therapy will decrease the risk of overlap in normal organs during treatment planning for recurrent disease.
- 5.
Intensity-modulated proton therapy is indicated for intracranial brain tumors and tumors near the skull base, such as chordoma [CR]
Scientific evidence
- 1.
Shih HA, Sherman JC, Nachtigall LB, et al. Proton therapy for low-grade gliomas: results from a prospective trial. Cancer. 2015 121(10):1712-1719.
- 2.
Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810-818.
- 3.
Brown AP, Barney CL, Grosshans DR, et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys. 2013;86(2):277-284.
- 4.
Farnia B, Allen PK, Brown PD, et al. Clinical outcomes and patterns of failure in pineoblastoma: a 30-year, single-institution retrospective review. World Neurosurg. 2014; 82(6):1232-1241.
- 5.
Barney CL, Brown AP, Grosshans DR, et al. Technique, outcomes, and acute toxicities in adults treated with proton beam craniospinal irradiation. Neuro Oncol. 2014;16(2):303-309.
- 6.
Arvold ND, Lessell S, Bussiere M, et al. Visual outcome and tumor control after conformal radiotherapy for patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2009;75(4):1166-1172.
- 7.
Hug EB, Devries A, Thornton AF, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48(2):151-160.
- 8.
Speirs CK, Simpson JR, Robinson CG, et al. Impact of 1p/19q codeletion and histology on outcomes of anaplastic gliomas treated with radiation therapy and temozolomide. Int J Radiat Oncol Biol Phys. 2015;91(2):268-276.
- 9.
Brown AP, Barney CL, Grosshans DR, et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys. 2013;86(2):277-284.
- 10.
Kabolizadeh P, Chen YL, Liebsch N, et al. Updated outcome and analysis of tumor response in mobile spine and sacral chordoma treated with definitive high-dose photon/proton radiation therapy. Int J Radiat Oncol Biol Phys. 2017;97(2):254-262.
- 11.
Weber DC, Ares C, Villa S, et al. Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: a phase-II parallel non-randomized and observation study (EORTC 22042-26042). Radiother Oncol. 2018;128(2):260-265.
- 12.
Cairncross G, Wang M, Jenkins R, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3): 337-343.
- 13.
Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374:1344-1355.
- 14.
Moeller BJ, Chintagumpapa M, Philip JJ, et al. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiat Oncol. 2011;6:58.
Recommended esophagus cancer proton therapy indications
Indications
- 1)
Stage I–III esophageal cancer
- ■
Proton therapy had clinical benefit in terms of postoperative morbidity and hospitalization in a multinational study comparing protons and intensity-modulated photon therapy for esophageal cancer after accounting for patient, tumor, and treatment factors. [CR]
- i.
Postoperative overall complications reduced by 40%.
- ii.
Postoperative pulmonary complications reduced by 47%.
- iii.
Postoperative cardiac complications reduced by 48%.
- iv.
Postoperative wound complications reduced by 75%.
- v.
Postoperative length of hospitalization reduced from a mean of 12.4 days (photons) to 9.2 days (proton).
- vi.
Postoperative 90-day mortality: 4.3% (photon) versus 0.9% (proton).
- i.
- ■
The relative benefits of proton therapy compared with the best intensity-modulated radiation therapy (IMRT) plans are based on the following systematic evaluation of a 55-patient cohort study [CR] :
- i.
Mean lung dose reduced by at least 30%.
- ii.
Lung volume of 5 Gy reduced by 45%, volume of 10 Gy reduced by 30%, and volume of 20 Gy reduced by 20% to 5%.
- iii.
Mean heart dose reduced by at least 35%.
- iv.
Mean liver dose reduced by 70% (for mid to distal esophageal tumors).
- v.
Maximum spinal cord hot spot dose reduced by 20%.
- i.
- ■
Proton therapy significantly spares heart and cardiac substructures in a large cohort of esophageal cancer [CR]
- i.
Compared with IMRT, proton beam therapy (PBT) resulted in significantly lower mean heart dose (MHD) and heart V5, V10, V20, V30, and V40, along with lower radiation exposure to the four chambers and four coronary arteries.
- ii.
Compared with passively scattered proton therapy (PSPT), intensity-modulated proton therapy (IMPT) resulted in significantly lower heart V20, V30, and V40 but not MHD or heart V5 or V10. IMPT also resulted in significantly lower radiation doses to the left atrium, right atrium, left main coronary artery, and left circumflex artery, but not the left ventricle, right ventricle, left anterior descending artery, or right coronary artery. Factors associated with lower MHD included PBT ( P < .001).
- i.
- ■
Definitive chemoradiation using proton beam therapy versus IMRT for esophageal cancer improves survival outcomes [CR]
- i.
Compared with IMRT, PBT had significantly better overall survival (OS; P = .011), progression-free survival (PFS; P = .001), distant metastasis-free survival (DMFS; P = .031), as well as marginally better locoregional failure-free survival (LRFFS; P = .075).
- ii.
No significant differences in rates of treatment-related toxicities were observed between groups.
- iii.
On multivariate analysis, IMRT had worse OS (hazard ratio [HR]: 1.454; P = .01), PFS (HR: 1.562; P = .001), and LRFFS (HR: 1.461; P = .041) than PBT. Subgroup analysis by clinical stage revealed considerably higher 5-year OS (34.6% vs. 25.0%; P = .038) and PFS rates (33.5% vs. 13.2%; P = .005) in the PBT group for patients with stage III disease.
- i.
- ■
Inclusion criteria
- i.
All resectable or unresectable esophageal cancer patients with nonmetastatic esophageal cancers.
- ii.
Adenocarcinoma or squamous cell carcinoma.
- iii.
Eligible to receive definitive or preoperative chemoradiation.
- iv.
Involves any part of the proximal thoracic to distal esophagus, the gastroesophageal junction, and the cardia of the stomach.
- i.
- ■
- 2)
IMPT is indicated for esophageal cancer.
Scientific evidence
- 1.
Lin SH, Merrell KW, Shen J, et al. Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother Oncol. 2017;123(3):376-381.
- 2.
Shiraishi Y, Xu C, Yang J, Komaki R, Lin SH. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or intensity-modulated radiation therapy. Radiother Oncol. 2017;125(1):48-54.
- 3.
Xi M, Xu C, Liao Z, et al. Comparative outcomes after definitive chemoradiotherapy using proton beam therapy versus intensity-modulated radiation therapy for esophageal cancer: a retrospective single-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;99(3):667-676.
- 4.
Lin SH, Komaki R, Liao Z, et al. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83(3):e345-e351.
- 5.
Ling TC, Slater JM, Nookala P, et al. Analysis of intensity-modulated radiation therapy (IMRT), proton and 3D conformal radiotherapy (3D-CRT) for reducing perioperative cardiopulmonary complications in esophageal cancer patients. Cancers (Basel). 2014;6(4):2356-2368.
- 6.
Mizumoto M, Sugahara S, Nakayama H, et al. Clinical results of proton-beam therapy for locoregionally advanced esophageal cancer. Strahlenther Onkol. 2010;186(9):482-488.
- 7.
Mizumoto M, Sugahara S, Okumura T, et al. Hyperfractionated concomitant boost proton beam therapy for esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e601-e606.
- 8.
Pan X, Zhang X, Li Y, Mohan R, Liao Z. Impact of using different four-dimensional computed tomography data sets to design proton treatment plans for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2009;73(2):601-609.
- 9.
Sugahara S, Tokuuye K, Okumura T, et al. Clinical results of proton beam therapy for cancer of the esophagus. Int J Radiat Oncol Biol Phys. 2005;61(1):76-84.
- 10.
Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2013;86(5):885-891.
- 11.
Wang SL, Liao Z, Vaporciyan AA, et al. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys. 2006;64(3):692-699.
- 12.
Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70(3):707-714.
- 13.
Welsh J, Gomez D, Palmer MB, et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: a dosimetric study. Int J Radiat Oncol Biol Phys. 2011;81(5):1336-1342.
- 14.
Zhang X, Zhao KL, Guerrero TM, et al. Four-dimensional computed tomography-based treatment planning for intensity-modulated radiation therapy and proton therapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2008;72(1):278-287.
- 15.
Wang J, Palmer M, Bilton SD, et al. Comparing proton beam to intensity-modulated radiation therapy planning in esophageal cancer. Int J Particle Ther. 2015;1(4):866-877.
Recommended gastrointestinal cancer proton therapy indications
Indications
- 1)
Hepatocellular carcinoma
- ■
Proton therapy with ablative doses for inoperable disease leads to prolongation of survival. [CR] , [CR] , [CR] , [CR] , [CR] , [CR]
- ■
- 2)
Intrahepatic cholangiocarcinoma
- ■
Proton therapy with ablative doses for inoperable disease leads to prolongation of survival. [CR] , [CR]
- ■
- 3)
Isolated colorectal liver metastases when liver constraints cannot be met with intensity-modulated radiation therapy (IMRT).
- ■
If all known disease can be covered, it is curative. Patients with stable and responding disease have a better prognosis, but treatment of all liver-confined colorectal metastases prolongs overall survival (OS), and some will be cured.
- ■
SBRT to ablative doses results in 90% local control. [CR] , [CR]
- ■
Surgical series demonstrating curative results in metastatic colorectal cancer with liver-dominant disease. [CR] , [CR]
- ■
- ■
- 4)
Recurrent, previously irradiated disease
- 5)
Any patient younger than 40 years old with localized, surgically curable disease where radiation is indicated.
- 6)
Intensity-modulated proton therapy (IMPT) is recommended for the indications listed above only in conjunction with respiratory gating.
Inclusion criteria:
- 1.
Curative treatment: For indications 1 to 3, ablative doses must be given to provide curative treatment. Three-dimensional conformal radiation therapy (3DCRT) or IMRT may be used if lower doses are prescribed. There may be exceptions, but the appeals process can address them.
- 2.
Ablative doses:
- ■
70 Gy in 10 fractions
- ■
60 to 67.5 Gy in 15 fractions
- ■
62.5 to 75 Gy in 25 fractions
- ■
Scientific evidence
- 1.
Hong TS, Wo JY, Yeap BY, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016;34(5):460-468.
- 2.
Tao R, Krishnan S, Bhosale PR, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis . J Clin Oncol. 2016;34(3):219-226.
- 3.
Brouquet A, Abdalla EK, Kopetz S, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083-1090.
- 4.
Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127 (5 suppl 1):S189-S193.
- 5.
Bush DA, Kayali Z, Grove R, et al. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117: 3053-3059.
- 6.
Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer. 2011;117:4060-4069.
- 7.
Chiba T, Tokuuye K, Matsuzaki Y, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res. 2005;11:3799-3805.
- 8.
Dionisi F, Ben-Josef E. The use of proton therapy in the treatment of gastrointestinal cancers: liver. Cancer J. 2014;20(6):371-377.
- 9.
Fukumitsu N, Sugahara S, Nakayama H, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831-836.
- 10.
Hashimoto T, Tokuuye K, Fukumitsu N, et al. Repeated proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2006;65(1):196-202.
- 11.
Hata M, Tokuuye K, Sugahara S, et al. Proton beam therapy for aged patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2007;69(3):805-812.
- 12.
Hata M, Tokuuye K, Sugahara S, et al. Proton beam therapy for hepatocellular carcinoma patients with severe cirrhosis. Strahlenther Onkol. 2006;182(12):713-720.
- 13.
Hata M, Tokuuye K, Sugahara S, et al. Proton beam therapy for hepatocellular carcinoma with limited treatment options. Cancer. 2006;107(3):591-598.
- 14.
Hata M, Tokuuye K, Sugahara S, et al. Proton beam therapy for hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2005;104(4):794-801.
- 15.
Hong TS, DeLaney TF, Mamon HJ, et al. A prospective feasibility study of respiratory-gated proton beam therapy for liver tumors. Pract Radiat Oncol. 2014;4(5):316-322.
- 16.
Kawashima M, Furuse J, Nishio T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma . J Clin Oncol. 2005;23:1839-1846.
- 17.
Kim TH, Park JW, Kim YJ, et al. Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res Treat. 2015;47(1):34-45.
- 18.
Komatsu S, Fukumoto T, Demizu Y, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer. 2011;117(21):4890-4904.
- 19.
Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677-3683.
- 20.
Lee SU, Park JW, Kim TH, et al. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol. 2014;190(9):806-814.
- 21.
Ling TC, Kang JI, Bush DA, Slater JD, Yang GY. Proton therapy for hepatocellular carcinoma. Chin J Cancer Res. 2012;24(4):361-367.
- 22.
Mizumoto M, Tokuuye K, Sugahara S, et al. Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int J Radiat Oncol Biol Phys. 2008;71(2):462-467.
- 23.
Mizumoto M, Okumura T, Hashimoto T, et al. Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int J Radiat Oncol Biol Phys. 2011;81(4):1039-1045.
- 24.
Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. 2009;115:5499-5506.
- 25.
Petersen JB, Lassen Y, Hansen AT, Muren LP, Grau C, Høyer M. Normal liver tissue sparing by intensity-modulated proton stereotactic body radiotherapy for solitary liver tumours. Acta Oncol. 2011;50(6):823-828.
- 26.
Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572-1578.
- 27.
Skinner HD, Hong TS, Krishnan S. Charged-particle therapy for hepatocellular carcinoma. Semin Radiat Oncol. 2011;21(4):278-286.
- 28.
Sugahara S, Nakayama H, Fukuda K, et al. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185(12):782-788.
- 29.
Sugahara S, Oshiro Y, Nakayama H, et al. Proton beam therapy for large hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2010;76(2):460-466.
- 30.
Taddei PJ, Howell RM, Krishnan S, Scarboro SB, Mirkovic D, Newhauser WD. Risk of second malignant neoplasm following proton versus intensity-modulated photon radiotherapies for hepatocellular carcinoma. Phys Med Biol. 2010;55(23):7055-7065.
- 31.
Toramatsu C, Katoh N, Shimizu S, et al. What is the appropriate size criterion for proton radiotherapy for hepatocellular carcinoma? A dosimetric comparison of spot-scanning proton therapy versus intensity-modulated radiation therapy. Radiat Oncol. 2013;8:48.
- 32.
Qi WX, Fu S, Zhang Q, Guo XM. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2014;114(3):289-295.
Recommended head and neck cancer proton therapy indications
Indications
- 1)
Nasal cavity and paranasal sinus tumors
- ■
Inclusion criteria
- i.
Ethmoid sinus tumors
- a.
Histologies include sinonasal undifferentiated carcinoma (SNUC), small cell neuroendocrine carcinoma, sarcoma, lymphoma
- b.
T1–T4a: Newly diagnosed, nonsurgical candidate or refuses surgery
- c.
T2–T4a: Postoperative with perineural invasion (PNI), bone invasion, or positive margin
- d.
T4b: Newly diagnosed or patient declines surgery
- e.
Diagnosed after incomplete resection and gross residual disease
- f.
Doses to optic apparatus structures exceed tolerance with photon therapy and may lead to blindness
- g.
No evidence of metastatic disease
- h.
Reirradiation in a site of recurrent disease
- i.
Reirradiation in a site of secondary primary
- a.
- ii.
Paranasal sinus tumors
- a.
Histologies include squamous cell carcinoma, adenocarcinoma, minor salivary gland tumor, esthesioneuroblastoma, undifferentiated carcinoma, sarcoma, mucosal melanoma, lymphoma
- b.
T1–T2: Postoperative with PNI, bone invasion, or positive margin
- c.
T1–T4a: Newly diagnosed, nonsurgical candidate or refuses surgery
- d.
T2–T4a: Postoperative with PNI, bone invasion, or positive margin
- e.
T4b: Newly diagnosed or patient declines surgery
- f.
Diagnosed after incomplete resection and gross residual disease
- g.
Doses to optic apparatus structures exceed tolerance with photon therapy and may lead to blindness
- h.
No evidence of metastatic disease
- i.
Reirradiation in a site of recurrent disease
- j.
Reirradiation in a site of secondary primary
- a.
- i.
- ■
For nasal cavity and paranasal sinus tumors, the preferred treatment algorithm includes surgery followed by postoperative external beam radiation therapy (EBRT). Even with this potentially disfiguring therapy, cure rates are poor, and treatment-related toxicities are common, severe, and can even be fatal. Control rates as low as 30% prompted investigation into new methods of treatment, particularly when tumors were situated near the skull base, and critical structures limit effective dose delivery to the tumor. Conventional EBRT to tumors of the paranasal sinuses has resulted in 24% to 41% incidence of blindness as a result of retinopathy or optic neuropathy. Treatment plans for proton beam radiation (PBR) compare favorably to photon EBRT, techniques such as intensity-modulated radiation therapy (IMRT) or 3D conformal radiation. A systemic review and metaanalysis by Patel et al. [CR] demonstrated a greater overall survival (OS) at 5 years with charged particle therapy over photon therapy and a higher disease-free survival and locoregional control (LRC) with proton therapy over IMRT. Investigators at the Massachusetts General Hospital (MGH) have had extensive experience with using proton therapy for nasal cavity and paranasal sinus cancer. In one such study, of 102 patients were treated between 1991 and 2002 with either PBR or combination proton-photon EBRT for locally advanced sinonasal cancers either with or without surgery. [CR] Although that analysis showed that a complete resection improved DFS and OS, high-dose radiation provided excellent local control (LC) regardless of the extent of resection. Another MGH study of 23 patients with adenoid cystic carcinomas that invaded the skull base treated with combined proton-photon RT reported a 5-year LC of 93%, despite the fact that 87% of patients had gross tumor remaining at the time of radiation, and 48% of patients received biopsy only before RT. [CR] The 5-year rates of freedom from distant metastasis were 62%; disease-free survival (DFS), 56%; and OS, 77%. These studies show good local control can be achieved with dose escalation using combined photon EBRT and PBR. The median doses used were 71.6 CGE and 75.9 CGE, respectively. However, the radiation delivery schedules were quite heterogeneous, with patients receiving conventional fractionation or twice-daily accelerated fractionation with or without concomitant boost. Truong et al. [CR] published the MGH experience with PBR for locally advanced tumors of the sphenoid sinus. This group was also heterogeneous in terms of histology, extent of surgery, and receipt of chemotherapy. Some patients also received photon radiation therapy for the neck, if indicated by histology. At 2 years, the LC rate was 86% the rate of freedom from metastases was 50%, the DFS rate was 31%, and the OS rate was 53%. In an early MGH experience with 19 prospectively treated patients with neuroblastoma and neuroendocrine tumors of the paranasal sinus, the patients underwent either biopsy, subtotal resection, or gross total resection with positive margins and then went on to receive two cycles of cisplatin-etoposide followed by combination photon and proton radiation therapy. [CR] A large field was treated with 1.8 Gy once daily using photons. A smaller field was supplemented with 1.6 CGE using protons on a twice-daily basis. The LC rate was good, with only two patients experiencing recurrence in the radiation field, both of which could be salvaged with surgery. No patients experienced radiation-induced vision loss, potentially because the chiasm and optic nerves were constrained to less than 55 CGE or less than 2 CGE per day and a stereotactic setup was used. At the Proton Medical Research Center in Tsukuba, Japan, 17 patients with T4 or recurrent nasal cavity or paranasal sinus carcinoma were treated with PBR to a median dose of 78 CGE in 36 fractions (range: 22–82.5 CGE). [CR] The 2- and 5-year LC were 35% and 18%, respectively, and the 2- and 5-year OS were 47% and 16%, respectively. Four patients experienced grade 3 to 4 toxicity, but there were no treatment-related deaths, potentially because of the strict dose constraint of 50 CGE to both the brainstem and optic chiasm. Another Japanese group used PBR for unresectable head and neck malignancies including nasal cavity and paranasal sinus or skull base tumors. [CR] The median dose of PBR was 65 CGE in 26 fractions, and 10 patients received neoadjuvant chemotherapy. At 1 year, they reported a 77% LC rate, with 3-year PFS and OS rates of 50% and 60%, respectively. The 5-year OS rate was 55%. In contrast to the MGH experience, however, treatment-related toxicities were not trivial. Cerebrospinal fluid leakage caused treatment-related death in one patient, and four other patients experienced grade 3 to 4 toxicities, including cataract, visual impairment, cranial nerve palsy, and osteonecrosis. However, because none the patients in this retrospective review were candidates for surgery, the inability to achieve LC of tumors in this area may very well have led to similar symptoms, and thus, the authors found this safety profile acceptable. With further improvements in technology to deliver PBR, such as intensity-modulated proton therapy (IMPT), the incidence of severe toxicities should be even lower in the future.
- ■
- 2)
Nasopharyngeal tumors
- ■
Inclusion criteria :
- i.
Histology including:
- ii.
Carcinoma (World Health Organization [WHO] I–III), adenoid cystic carcinoma, sarcoma, lymphoma
- iii.
T1–T4: Definitive treatment with radiation therapy alone or with concurrent chemotherapy
- i.
- ■
Lin et al. [CR] presented outcomes for patients with recurrent nasopharyngeal carcinoma initially treated at Loma Linda University Medical Center (LLUMC) with photon radiation therapy who were then reirradiated to doses of 59.4 to 70.2 CGE. Their OS and locoregional PFS were both 50% at 2 years but varied widely by how well the target was covered. Dose-volume histograms were analyzed for “optimal” (2-year OS: 83%) versus “suboptimal coverage” (2-year OS: 17%), where “optimal” was conservatively defined as 90% of the target volume receiving 90% of the prescribed dose Chan et al. [CR] reported outcomes of 19 patients with T4 nasopharyngeal cancer treated with conformal photon-proton radiation therapy to a medial total dose of 73.6 CGE in either twice-daily or conventional fractionation. Ten patients also received induction or concurrent chemotherapy with docetaxel, cisplatin, carboplatin, or paclitaxel. Three-year rates of LC, PFS, and OS were 92%, 75%, and 74%, respectively, with one patient hospitalized for acute toxicities and five patients noted to have late toxicities, including temporal lobe radiographic changes, mandibular osteonecrosis, and endocrine dysfunction. A phase II study at MGH evaluated combined proton-photon radiation therapy to 70 CGE in 35 daily fractions given with concurrent cisplatin and fluorouracil (NCT00592501). The primary outcomes are acute toxicity, treatment compliance, and health-related quality of life at 3 years. Preliminary results from 23 consecutive patients with stage III to IVB nasopharyngeal cancer were presented at the 2014 meeting of the American Society for Radiation Oncology. [CR] The LRC rate at 28 months was 100%; 2-year DFS and OS rates were 90% and 100%, respectively. Toxicities included hearing loss in 29%, weight loss in 38%, and gastrostomy tube placement in 48% but no grade 3 or greater xerostomia.
- ■
- 3)
Oropharyngeal tumors
- ■
Inclusion criteria
- i.
Histologies include squamous cell carcinoma, adenoid cystic carcinoma, sarcoma
- ii.
T1–T4a: Definitive treatment with radiation therapy alone or with concurrent chemotherapy
- iii.
T1–T4a: Postoperative with high-risk features (i.e., positive margins, extracapsular extension [ECE], PNI)
- iv.
Coverage of the retropharyngeal nodes required to the skull base
- v.
T4b: unresectable advanced stage disease with the treatment volume extending to the skull base
- vi.
Unknown primary with cervical nodal disease requiring coverage of the pharyngeal axis to the skull base
- i.
- ■
Oropharyngeal cancer is often treated with radiation therapy alone or in combination with chemotherapy. In the 10 years since this book was written, IMRT has become the standard treatment technique for head and neck malignancies because of its ability to reduce dose to the parotids and minimize the risk of xerostomia. However, additional dose is now delivered to the oral cavity, larynx, brainstem, and muscle of mastication that can cause dysgeusia (loss of taste), dysphagia, xerostomia, and trismus. The unnecessary radiation dose to the head and neck outside of the tumor from IMRT results in nausea, vomiting, anterior oral mucositis, oral pain, dysphagia, fatigue, loss of weight, gastrostomy tubes, emergency room visits with intravenous (IV) fluids, and hospitalization. After LLUMC opened the first proton treatment facility in the hospital-based environment in 1990, they opened a prospective protocol to treat patients with stage II–IV oropharyngeal cancer with a combination of photon and proton therapy. In 2005, Slater et al. [CR] published the LLUMC experience using a concomitant boost technique, which consisted of 50.4 Gy in 1.8-Gy fractions to the primary disease volume, involved lymph nodes, and areas at risk with lateral opposed photon fields. Concomitant boost proton fields encompassed gross primary and nodal disease, which was delivered during the last 3.5 weeks of photon treatment in a twice-daily fashion, bringing the total tumor dose to 75.9 CGE. They achieved 92% 2-year LRC compared with 55% in the Radiation Therapy Oncology Group (RTOG) concomitant boost arm with standard radiation therapy, suggesting that higher doses could improve outcomes without an increase in toxicity. In an MD Anderson case-control study by Frank et al. [CR] of IMPT versus IMRT, IMPT-treated patients had a gastrostomy tube rate of 19% compared with an IMRT rate of 46% and a lower rate of grade 3 dysphagia. The most recent data from MD Anderson on the first 50 patients treated with IMPT and a median follow-up of 25 months demonstrates one local treatment failure and one regional treatment failure with no grade 4 or 5 toxicities. [CR]
- ■
- 4)
Periorbital tumors
- ■
Inclusion criteria
- i.
Medial canthal tumors (i.e., squamous cell carcinoma, basal cell carcinoma, Merkel cell carcinoma)
- ii.
Tumors of the lacrimal sac/duct
- iii.
Lacrimal gland tumors
- iv.
Eyelid tumors
- v.
Histologies include squamous cell carcinoma, basal cell carcinoma, adenoid cystic carcinoma, sarcoma
- vi.
T1–T4a: Definitive treatment with radiation therapy alone or with concurrent chemotherapy
- vii.
T1–T4a: Postoperative with high-risk features (i.e., positive margins, ECE, PNI)
- viii.
T4b: Unresectable advanced stage disease with the treatment volume extending to the skull base
- ix.
Orbit-sparing approach to prevent blindness
- i.
- ■
For orbit-sparing approaches to preserve the eye from enucleation or total exenteration, proton therapy is necessary to avoid long-term ophthalmologic complications that can affect many portions of the eye, including the lacrimal glands, eyelids, conjunctiva, sclera, cornea, lens, retina, optic nerves, and optic chiasm. Complications from radiation therapy include decreased tear production and dry eye, lacrimal duct atrophy and epiphora, eyelid or corneal ulceration, telangiectasis, conjunctiva neovascularization, keratinization, cataracts, glaucoma, retinopathy, and optic neuropathy.
- ■
Medial canthal tumors (i.e., squamous cell carcinoma, basal cell carcinoma, Merkel cell carcinoma)
- ■
Tumors of the lacrimal sac/duct
- ■
Lacrimal gland tumors
- ■
Eyelid tumors
- ■
- ■
- 5)
Skull-base tumors
- ■
Inclusion criteria
- i.
Histologies at the skull base include, paragangliomas/schwannomas, salivary gland tumors, chordomas and chondrosarcomas, sarcomas, squamous cell carcinoma, and adenoid cystic carcinoma.
- ii.
T1–T2: Postoperative and high-risk features (PNI, grade, positive margins)
- iii.
T3–T4a: Patient declines surgery or disease is medically inoperable
- iv.
T2–T4a: Postoperative with high-risk features (PNI, positive margins)
- v.
T4b: No surgical resection possible or surgical resection not recommended
- vi.
No evidence of distant metastases
- vii.
Skin primary with perineural spread into the skull base via foramen rotundum, ovale, or Meckel’s cave toward the cavernous sinus
- i.
- ■
For tumors arising at the base of the skull, complete resection is often impossible. Unfortunately, the relative radioresistance of these tumors makes treatment with a curative dose difficult without exposing the surrounding tissues to unacceptable toxicity. Treatment with photon EBRT leads to high recurrence rates and a 5-year progression-free survival (PFS) rate of less than 25%. Doses of 60 Gy and lower were equally ineffective. Even though doses given were inadequate for durable LC, significant brainstem and cranial nerve toxicities were seen with doses of 60 Gy.
- ■
Paragangliomas/schwannomas at the skull base
- ■
Salivary gland tumors at the skull base
- ■
Chordomas and chondrosarcomas at the skull base
- ■
Sarcomas and carcinomas at the skull base
- ■
- ■
- 6)
Reirradiation for recurrent head and neck tumors or new primary malignancies in a radiated field
- 7)
IMPT is indicated for all head and neck malignancies.
General inclusion criteria
- 1.
Curative primary and adjuvant treatment where the tumor and target radiation volume extends to the base of the skull
- ■
For any of these indications where doses of 60 to 70 Gy are required for curative intent, IMRT may be used if lower doses are prescribed. There may be exceptions, but the appeals process can address them.
- ■
- 2.
Advanced-stage head and neck disease where concurrent chemotherapy is required to control the local or regional disease
- 3.
Periorbital tumors where blindness, enucleation, or total exenteration are a risk from standard radiation therapy
- 4.
Reirradiation in the head and neck where cumulative doses exceeding 100 Gy may result in significant acute and long-term toxicity and death from treatment
General exclusion criteria (or peer to peer required for specific special circumstances):
- 1.
Cancer of the larynx, unless carotid artery sparing not achievable with IMRT or specific histology with unresectable disease such as adenoid cystic carcinoma.
- 2.
Cancer of the skin without PNI requiring treatment coverage to the skull base.
- 3.
Cancer of the lip without PNI requiring treatment coverage to the skull base.
- 4.
Mucosal melanoma without skull base involvement.
Scientific evidence
- 1.
Colevas AD, Yom SS, Pfister DG, et al. NCCN Guidelines Head and Neck Cancers-Version 2.2018, June 20, 2018. J Natl Compr Cancer Netw. 2018;16(5).
- 2.
Holliday EB, Frank SJ. Proton radiation therapy for head and neck cancer: a review of the clinical experience to date. Int J Radiat Oncol Biol Phys. 2014;89(2):292-302.
- 3.
Patel SH, Wang Z, Wong WW, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15(9):1027-1038.
- 4.
Resto VA, Chan AW, Deschler DG, Lin DT. Extent of surgery in the management of locally advanced sinonasal malignancies. Head Neck 2008;31(2):222-229.
- 5.
Pommier P, Liebsch NJ, Deschler DG, et al. Proton beam radiotherapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg 2006;132(11):1242-1249.
- 6.
Truong MT, Kamat UR, Liebsch NJ, et al. Proton radiotherapy for primary sphenoid sinus malignancies: treatment outcomes and prognostic factors. Head Neck 2009;31(10):1297-1308.
- 7.
Fitzek MM, Thornton AF, Varvares M, et al. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer . 2002;94:2623-2634.
- 8.
Okano S, Tahara M, Zenda S, et al. Induction chemotherapy with docetaxel, cisplatin and S-1 followed by proton beam therapy concurrent with cisplatin in patients with T4b nasal and sinonasal malignancies. Jpn J Clin Oncol. 2012;42:691-696.
- 9.
Zenda S, Kohno R, Kawashima M, et al. Proton beam therapy for unresectable malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys. 2011;81:1473-1478.
- 10.
Lavertu P, Roberts JK, Kraus DH, et al. Squamous cell carcinoma of the paranasal sinuses: the Cleveland Clinic experience 1977–1986. Laryngoscope . 1989;99:1130-1136.
- 11.
Waldron JN, O’Sullivan B, Warde P, et al. Ethmoid sinus cancer: twenty-nine cases managed with primary radiation therapy. Int J Radiat Oncol Biol Phys. 1998;41:361-369.
- 12.
Takeda A, Shigematsu N, Suzuki S, et al. Late retinal complications of radiation therapy for nasal and paranasal malignancies: relationship between irradiated-dose area and severity. Int J Radiat Oncol Biol Phys. 1999;44:599-605.
- 13.
Katz TS, Mendenhall WM, Morris CG, et al. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck . 2002;24:821-829.
- 14.
Lomax AJ, Goitein M, Adams J. Intensity modulation in radiotherapy: Photons versus protons in the paranasal sinus. Radiother Oncol. 2003;66:11-18.
- 15.
Mock U, Georg D, Bogner J, et al. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:147-154.
- 16.
Fukumitsu N, Okumura T, Mizumoto M, et al. Outcome of T4 (International Union Against Cancer Staging System, 7th edition) or recurrent nasal cavity and paranasal sinus carcinoma treated with proton beam. Int J Radiat Oncol Biol Phys. 2012;83:704-711.
- 17.
Lin R, Slater JD, Yonemoto LT, et al. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy—dose-volume histogram analysis. Radiology . 1999;213:489-494.
- 18.
Chan A, Liebsch L, Deschler D, et al. Proton radiotherapy for T4 nasopharyngeal carcinoma. J Clin Oncol. 2004;22:5574.
- 19.
Chan A, Adams JA, Weyman E, et al. A phase II trial of proton radiation therapy with chemotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:S151-S152.
- 20.
Kam MKM, Leung S-F, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873-4879.
- 21.
Liu S-W, Li J-M, Chang J-Y, et al. A treatment planning comparison between proton beam therapy and intensity-modulated x-ray therapy for recurrent nasopharyngeal carcinoma. J X-Ray Sci Technol. 2010;18:443-450.
- 22.
Taheri-Kadkhoda Z, Björk-Eriksson T, Nill S, et al. Intensity-modulated radiotherapy of nasopharyngeal carcinoma: a comparative treatment planning study of photons and protons. Radiat Oncol. 2008;3:4.
- 23.
Slater JD, Yonemoto LT, Mantik DW, et al. Proton radiation for treatment of cancer of the oropharynx: early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys. 2005;62:494-500.
- 24.
Frank SJ, Rosenthal DI, Ang K, et al. Gastrostomy tubes decrease by over 50% with intensity modulated proton therapy (IMPT) during the treatment of oropharyngeal cancer patients: a case-control study. Int J Radiat Oncol Biol Phys . 2013;87(2):S144.
- 25.
Kutcheson K, Lewin JS, Garden AS, et al. Early experience with IMPT for the treatment of oropharyngeal tumors: acute toxicities and swallowing-related outcomes. Int J Radiat Oncol Biol Phys . 2013;87:S604.
- 26.
Parsons JT, Bova FJ, Fitzgerald CR, et al. Severe dry-eye syndrome following external beam irradiation. Int J Radiat Oncol Biol Phys . 1994;30:775-780.
- 27.
Barabino S, Raghavan A, Loeffler J, et al. Radiation therapy-induced ocular surface disease. Cornea . 2005;24:909-914.
- 28.
Van de Water TA, Bijl HP, Schilstra C, et al. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist . 2011;16:366-377.
- 29.
Catton C, O’Sullivan B, Bell R, et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol. 1996;41:67-72.
- 30.
Frank SJ, Cox JD, Gillin M, et al. Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice. Int J Radiat Oncol Biol Phys. 2014;89:846-853.
- 31.
Gunn GB, Blanchard P, Garden AS, et al. Clinical outcomes and patterns of disease recurrence after intensity modulated proton therapy for oropharyngeal squamous carcinoma. Int J Radiat Oncol Biol Phys. 2016;95:360-367.
- 32.
Holliday EB, Kocak-Uzel E, Feng L, et al. Dosimetric advantages of intensity-modulated proton therapy for oropharyngeal cancer compared with intensity-modulated radiation: a case-matched control analysis. Med Dosim. 2016;41:189-194.
- 33.
Eekers DBP, Roelofs E, Jelen U, et al. Benefit of particle therapy in re-irradiation of head and neck patients. Results of a multicentric in silico ROCOCO trial. Radiother Oncol. 2016;121:387-394.
- 34.
Sapir E, Tao Y, Feng F, et al. Predictors of dysgeusia in patients with oropharyngeal cancer treated with chemotherapy and intensity modulated radiation therapy. Int J Radiat Oncol. 2016;96:354-361.
- 35.
Jakobi A, Bandurska-Luque A, Stützer K, et al. Identification of patient benefit from proton therapy for advanced head and neck cancer patients based on individual and subgroup normal tissue complication probability analysis. Int J Radiat Oncol Biol Phys. 2015;92:1165-1174.
- 36.
Blanchard P, Wong AJ, Gunn GB, et al. Toward a model-based patient selection strategy for proton therapy: external validation of photon- derived normal tissue complication probability models in a head and neck proton therapy cohort. Radiother Oncol. 2016;121:381-386.
- 37.
Blanchard P, Garden AS, Gunn GB, et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer—a case matched analysis. Radiother Oncol. 2016;120:48-55.
- 38.
Sio TT, Lin HK, Shi Q, et al. Intensity modulated proton therapy versus intensity modulated photon radiation therapy for oropharyngeal cancer: first comparative results of patient-reported outcomes. Int J Radiat Oncol Biol Phys. 2016;95:1107-1114.
- 39.
Romesser PB, Cahlon O, Scher ED, et al. Proton beam reirradiation for recurrent head and neck cancer multi-institutional report on feasibility and early outcomes. Int J Radiat Oncol Biol Phys. 2016;95:386-395.
- 40.
Phan J, Sio TT, Nguyen TP, et al. Reirradiation of head and neck cancers with proton therapy: outcomes and analyses. Int J Radiat Oncol Biol Phys. 2016;96:30-41.
- 41.
Esmaeli B, Yin VT, Hanna EY, et al. Eye-sparing multidisciplinary approach for the management of lacrimal gland carcinoma. Head Neck. 2016;38:1258-1262.
- 42.
Holliday EB, Esmaeli B, Pinckard J, et al. A multidisciplinary orbit-sparing treatment approach that includes proton therapy for epithelial tumors of the orbit and ocular adnexa. Int J Radiat Oncol Biol Phys. 2016;95:344-352.
- 43.
Bhattasali O, Holliday E, Kies MS, et al. Definitive proton radiation therapy and concurrent cisplatin for unresectable head and neck adenoid cystic carcinoma: a series of 9 cases and a critical review of the literature. Head Neck. 2016;38(suppl 1):E1472-E1480.
- 44.
Romesser PB, Cahlon O, Scher E, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016;118:286-292.
- 45.
Thaker NG, Frank SJ, Feeley TW. Comparative costs of advanced proton and photon radiation therapies: lessons from time-driven activity-based costing in head and neck cancer. J Comp Eff Res. 2015;4:297-301.
- 46.
Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost- effectiveness studies of proton radiotherapy. Cancer. 2016;122:1483-1501.
- 47.
Russo AL, Adams JA, Weyman EA, et al. Long-term outcomes after proton beam therapy for sinonasal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2016;95:368-376.
- 48.
Dagan R, Bryant C, Li Z, et al. Outcomes of sinonasal cancer treated with proton therapy. Int J Radiat Oncol Biol Phys. 2016;95:377-385.
- 49.
Holliday EB, Garden AS, Rosenthal DI, et al. Proton therapy reduces treatment-related toxicities for patients with nasopharyngeal cancer: a case-match control study of intensity-modulated proton therapy and intensity modulated photon therapy. Int J Part Ther. 2015;2:19-28.
- 50.
McDonald MW, Zolali-Meybodi O, Lehnert SJ, et al. Reirradiation of recurrent and second primary head and neck cancer with proton therapy. Int J Radiat Oncol Biol Phys. 2016;96:808-819.
Recommended hematologic cancer proton therapy indications
Indications
- 1)
Mediastinal lymphoma
- ■
Inclusion criteria
- i.
Lymphoma located in the mediastinum with the following pathologies: Hodgkin lymphoma, diffuse large B-cell, lymphoblastic lymphoma, lymphoblastic leukemia of T, B, pre-T, or pre-B type, and primary mediastinal lymphoma
- ii.
Lymphoma located behind the heart
- iii.
Lymphoma in a patient who previously received radiation to the same area
- i.
- ■
For this young population that survives for decades, treating the critical organs to even low doses will result in second malignancies and heart disease 15 to 25 years later.
- ■
Studies of radiation-related heart disease strongly suggest that increasing dose to the heart increases the risk of cardiac complications, [CR] , [CR] with some suggesting that there is no threshold below which there is no risk.
- ■
In addition, our goal is to decrease the volume of lung receiving a low radiation dose with proton therapy compared with intensity-modulated radiation therapy (IMRT). Our recent work showed that a mean lung dose of as low as 13 Gy is associated with a higher rate of pneumonitis. This is different than the threshold for other oncologic sites because most of our patients receive one or more types of chemotherapy that cause lung toxicity (examples include bleomycin, gemcitabine, busulfan, brentuximab).
- ■
- 2)
Craniospinal irradiation
- ■
Inclusion criteria
- i.
Definitive therapy OR
- ii.
Conditioning before allogeneic transplant
- i.
- ■
Our main goal is not to exit through critical organs primarily the vertebral bodies that contain more than 50% of the bone marrow (using proton therapy). Avoiding the marrow will prevent pancytopenia as well as permanent damage to a large part of the bone marrow that receives 30 Gy, which is the usual dose of radiation given.
- ■
In the setting of autologous or allogeneic transplant, proton therapy will help to avoid the following critical organs: lungs, heart, thyroid, and bowel. This will decrease the side effects to these organs and substantially reduce the hospital stay, transfusions, and infection.
- ■
- 3)
Lymphoma near the paraspinal region along the whole vertebral body
- ■
Inclusion criteria
- i.
Paraspinal masses of any type of lymphoma that need treatment with radiation as definitive therapy or as a consolidation
- ii.
Sacral masses of any type of lymphoma
- iii.
Posterior thoracic spinal masses that are located in the vicinity of the heart
- iv.
Sternal masses
- i.
- ■
Proton therapy will eliminate the risk of excess dose to the organs anterior to the mass, including thyroid, lungs, heart, bilateral kidneys, ovaries, and bowels
- ■
Will also avoid dose to the bone marrow
- ■
Scientific evidence
- 1.
Brenner H, Gondos A, Pulte D. Ongoing improvement in long-term survival of patients with Hodgkin disease at all ages and recent catch-up of older patients. Blood. 2008; 111(6):2977-2983.
- 2.
Carr ZA, Land CE, Kleinerman RA, et al. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys. 2005;61:842-850.
- a.
A statistically significant relationship was observed between coronary heart disease average dose to the heart in the 0 to 7.6 Gy range. The study is important in that it shows that even very low doses (≥2 Gy) may be associated with increased risk of coronary artery heart disease.
- a.
- 3.
Chera BS, Rodriguez C, Morris CG, et al. Dosimetric comparison of three different involved nodal irradiation techniques for stage II Hodgkin’s lymphoma patients: Conventional radiotherapy, intensity-modulated radiotherapy and three-dimensional proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(4):1173-1180.
- ■
Mean breast dose was highest (1.94 Gy) for conformal radiotherapy, 3.74 Gy for IMRT, 1.59 Gy for three-dimensional proton radiotherapy.
- ■
Mean lung doses: 4.83 Gy conformal radiotherapy, 5.38 Gy IMRT, and 30.4 Gy 3D proton therapy.
- ■
In general, the advantage for protons is seen in volume receiving relatively low dose (<15 Gy).
- ■
- 4.
Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;11:987-998.
- ■
Cardiac risk strongly related to cardiac dose with no obvious threshold.
- ■
- 5.
Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 1993;270(16):1949-1955.
- 6.
Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150(5):977-982.
- 7.
Heidenreich PA, Schnittger I, Strauss HW, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J Clin Oncol. 2007;25(1):43-49.
- 8.
Hoppe BS, Flampouri S, Zaiden R, et al. Involved-node proton therapy in combined modality therapy for Hodgkin lymphoma: results of a phase 2 study. Int J Radiat Oncol Biol Phys. 2014;89(5):1053-1059.
- a.
Progressively lower average integral dose and average dose to heart, lungs, breast, thyroid, and esophagus when 3D, IMRT, and proton plans were compared in 15 patients treated with involved-node proton therapy after chemotherapy. Three-year event-free survival rate 93%.
- a.
- 9.
Hoppe BS, Flampouri S, Su Z, et al. Effective dose reduction to cardiac structures using protons compared with 3DCRT and IMRT in mediastinal Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2012;84(2):449-455.
- a.
Highly significant decrease in dose with comparison of proton therapy versus 3D or IMRT to multiple critical organs with proton therapy, including heart, left ventricle, right ventricle, left atrium, mitral valve, tricuspid valve, aortic valve (significant only for 3D vs. proton therapy), left anterior descending artery, left circumflex, right circumflex (significant only for 3D vs. proton therapy), pulmonary artery (significant only for 3D vs. proton therapy), and ascending aorta (significant only for IMRT vs. proton therapy).
- a.
- 10.
Hoppe BS, Flampouri S, Su Z, et al. Consolidative involved-node proton therapy for stage IA–IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a phase II study. Int J Radiat Oncol Biol Phys. 2012;83(1):260-267.
- a.
“PT provided the lowest mean dose to the heart, lungs, and breasts for all 10 patients compared with either 3D-CRT or IMRT.”
- a.
- 11.
Jørgensen AY, Maraldo MV, Brodin NP, et al. The effect on esophagus after different radiotherapy techniques for early stage Hodgkin’s lymphoma. Acta Oncol. 2013;52: 1559-1565.
- a.
“Mean dose to the esophagus was 16.4 Gy with 3DCRT, 16.4 Gy with VMAT, 14.7, Gy with proton therapy and 34.2 Gy with mantle field treatment (P <0.001). No differences were seen in the estimated risk of developing esophagitis, stricture or cancer with 3DCRT compared with VMAT. Proton therapy performed significantly better with the lowest risk estimates for all parameters compared with the photon treatments, except compared with 3DCRT for stricture (P = 0.066).”
- a.
- 12.
Li J, Dabaja B, Reed V, et al. Rationale for and preliminary results of proton beam therapy for mediastinal lymphoma. Int J Radiat Oncol Biol Phys. 2011;81(1):167-174.
- a.
In 10 patients, “PBT delivered lower mean doses to the lung (6.2 vs. 9.5 Gy), esophagus (9.5 vs. 22.3 Gy), and heart (8.8 vs. 17.7 Gy) but not the breasts (5.9 vs. 6.1 Gy) than did conventional RT.”
- a.
- 13.
Maraldo MV, Brodin NP, Aznar MC, et al. Estimated risk of cardiovascular disease and secondary cancers with modern highly conformal radiotherapy for early-stage mediastinal Hodgkin lymphoma. Ann Oncol. 2013;24:2113-2118.
- a.
Compared with arc IMRT (VMAT) or 3D conventional therapy, highly significant estimated benefit for protons as measured by cardiac mortality, cardiac morbidity, myocardial infarction, valvular disease (only VMAT vs. PT significant), lung cancer, breast cancer, and life years lost.
- a.
- 14.
Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606.
- 15.
Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. Clin Oncol. 2002;20(8):2101-2108.
- 16.
Pinnix CC, Smith GL, Milgrom S, et al. Predictors of radiation pneumonitis in patients receiving intensity-modulated radiation therapy for Hodgkin and non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2015;92(1):175-182.
- 17.
Schneider U, Lomax A, Lombriser N. Comparative risk assessment of secondary cancer incidence after treatment of Hodgkin’s disease with photon and proton radiation. Radiat Res. 2000;154:382-388.
- a.
This is basically a case report in which the risk of secondary cancer is calculated for several different kinds of plans (two field photons, IMRT, and two different proton plans). “Irradiation with protons using the spot scanning technique decreases the avoidable cancer incidence compared with photon treatment by a factor of about two.”
- a.
- 18.
Seppenwoolde Y, Lebesque JV, de Jaeger K, et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2003;55:724-735.
- a.
The risk of radiation pneumonitis in 382 patients with breast cancer, lymphoma, and lung cancer was assessed in relation to a variety of measures of radiation dose to the lungs. The risk of pneumonitis was estimated to be more than 5% if the mean lung dose was greater than approximately 12 Gy or if the volume of lung receiving more than 13 Gy (V13) was more than 23%.
- a.
Recommended pediatric cancer proton therapy indications
Indications
- 1)
Curative treatment of pediatric brain, nonbrain solid, and hematologic tumors
The Stockholm Pediatric Proton Therapy Conference Consensus Report in 2016 provides recommendations on which pediatric cancer patients may benefit from proton therapy.
Inclusion criteria
- i.
Brain tumors, such as medulloblastoma, ependymoma, low-grade glioma, craniopharyngioma, germ cell tumor, atypical teratoid rhabdoid tumor, choroid plexus tumor, anaplastic oligodendroglioma, meningioma, and cases requiring craniospinal irradiation. Spinal tumors for which anterior thoracic or abdominopelvic organs would be spared from radiation.
- ii.
Solid tumors, such as rhabdomyosarcoma, nonrhabdomyosarcoma soft tissue sarcoma, Ewing sarcoma, osteosarcoma, retinoblastoma, neuroblastoma, Wilms and other renal tumors, germ cell tumors, nasopharyngeal carcinoma, salivary gland tumor, and other head and neck cancer.
- iii.
Hematologic tumors, such as Hodgkin lymphoma, extranodal, nasal-type natural killer lymphoma. Also, leukemia or lymphoma requiring craniospinal irradiation.
- i.
General exclusion criteria
- i.
Definitive treatment of noncurable cases such as diffuse intrinsic pontine glioma, malignant gliomas other than anaplastic oligodendroglioma
- ii.
Whole brain, whole lung, and whole abdominal radiotherapy where the target volume is purposely not sparing any critical structure
- iii.
Total body irradiation
- i.
- 2)
Reirradiation to spare critical structures/organs next to the target volume
Scientific evidence
- 1.
Antonini TN, Ris MD, Grosshans DR, et al. Attention, processing speed, and executive functioning in pediatric brain tumor survivors treated with proton beam therapy. Radiother Oncol. 2017;124(1):89-97.
- 2.
Bishop AJ, Greenfield B, Mahajan A, et al. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys. 2014;90(2):354-361.
- 3.
Eaton BR, Chowdhry V, Weaver K, et al. Use of proton therapy for re-irradiation in pediatric intracranial ependymoma. Radiother Oncol. 2015;116(2):301-308.
- 4.
Eaton BR, Esiashvili N, Kim S, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol. 2016;18(6):881-887.
- 5.
Greenfield BJ, Jaramillo S, Abboud M, et al. Outcomes for pediatric patients with central nervous system germ cell tumors treated with proton therapy. Clin Transl Radiat Oncol. 2016;1:9-14.
- 6.
Hattangadi JA, Rombi B, Yock TI, et al. Proton radiotherapy for high-risk neuroblastoma: early outcomes and dose comparison. Int J Radiat Oncol Biol Phys. 2012;83(3):1015-1022.
- 7.
Hess CB, Indelicato DJ, Paulino AC, et al. An update from the Pediatric Proton Consortium Registry. Front Oncol. 2018;8:165.
- 8.
Indelicato DJ, Merchant T, Laperriere N, et al. Consensus report from the Stockholm Pediatric Proton Therapy Conference. Int J Radiat Oncol Biol Phys. 2016;96(2):387-392.
- 9.
Kahalley LS, Ris MD, Grosshans DR, et al. Comparing intelligence quotient change after proton versus photon radiotherapy for pediatric brain tumors. J Clin Oncol. 2016; 34(10):1043-1049.
- 10.
Kamran SC, Goldberg SI, Kuhlthau KA, et al. Quality of life in patients with proton-treated medulloblastoma: results of a prospective assessment with 5 year follow-up. Cancer. 2018;124(16):3390-3400.
- 11.
Ladra MM, Szymonifka JD, Mahajan A, et al. Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol. 2014;32(33): 3762-3770.
- 12.
McGovern SL, Okcu MF, Munsell MF, et al. Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. Int J Radiat Oncol Biol Phys. 2014;90(5):1143-1152.
- 13.
Mouw KW, Yeap BY, Caruso P, et al. Analysis of patient outcomes following proton therapy for retinoblastoma. Adv Radiat Oncol. 2017;2(1):44-52.
- 14.
Pulsifer MB, Duncanson H, Grieco J, et al. Cognitive and adaptive outcomes after proton radiation for pediatric patients with brain tumors. Int J Radiat Oncol Biol Phys. 2018;102(2):391-398.
- 15.
Sato M, Gunther JR, Mahajan A, et al. Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer. 2017;123(13):2570-2578.
- 16.
Vern-Gross TZ, Indelicato DJ, Bradley JA, Rotondo RL. Patterns of failure in pediatric rhabdomyosarcoma after proton therapy. Int J Radiat Oncol Biol Phys. 2016;96(5): 1070-1077.
- 17.
Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 2016;17(3):287-298.
Recommended prostate cancer proton therapy indications
Indications
- 1)
Definitive/curative treatment for men with intact localized or locally advanced prostate cancer
- ■
Inclusion criteria
- i.
Men with T1-2N0M0 with Gleason sum 7 (or higher) or prostate-specific antigen (PSA) levels of 10 ng/mL (or higher) prostate cancer who have elected to proceed with external beam radiation therapy for definitive therapy with or without hormone therapy and have a projected life expectancy of at least 10 years
- a.
Doses ranges should be 76 to 80 Gy equivalents (GyE) at 1.8 to 2 GyE per fraction
- b.
Some men (e.g., those with reasonable urinary function) can be considered for hypofractionated regimens of 70 to 72 GyE at 2.4 to 2.5 GyE per fraction
- c.
Men with low-risk and select cases of intermediate-risk cancer (i.e., T1–2a, Gleason 7 in <50% cores, PSA <10) can be considered for clinical studies involving ultra-hypofractionation (e.g., 40 GyE at 8 GyE per fraction or 55.5 GyE at 3.7 GyE per fraction)
- a.
- ii.
Men with T3–4 and/or Gleason 8 to 10 and/or PSA over 20 disease for which higher radiation doses are needed to maximize local control
- a.
Conventional fractionation should typically be used (e.g., 76–82 GyE at 1.8–2 GyE per fraction)
- b.
Pelvic nodal radiation (e.g., 44–50 GyE) may also be considered in some cases
- c.
Given the increased target volumes and complexity of such cases, pencil beam proton delivery planning and delivery techniques may be required to optimize dosimetric constraints on normal tissues
- a.
- i.
- ■
Delivering a high radiation dose to the primary tumor in the prostate and/or seminal vesicles has become an important aspect of the optimal management of clinically localized prostate carcinoma. This is the result of multiple phase III studies demonstrating that a higher radiation dose reduces the risk of prostate cancer recurrence.
- ■
Proton therapy can be at least as effective as conventional external beam radiotherapy in treating clinically localized (encompassing low-risk, intermediate-risk, and high-risk) prostate cancer while reducing the risk of acute and late side effects of radiotherapy. When this book was written a phase III study (NCT 01617161) was in progress to compare proton therapy with conventional external beam radiotherapy using intensity-modulated radiotherapy (IMRT). Table 1 compares conventional external beam radiotherapy and proton therapy with respect to therapeutic effectiveness in terms of biochemical relapse-free rate, based on some of published manuscripts and abstracts. Biochemical relapse (also called PSA relapse ) is a widely accepted surrogate representing prostate cancer recurrence. Table 2 compares the incidences of acute and late radiation toxicity between conventional external beam radiotherapy and proton therapy. Patient-reported quality of life data from two prospectively collected databases also suggest an approximately 50% reduction in problems with significant bowel urgency and frequency in patients treated with proton therapy compared with IMRT.
- ■