Abstract
Vascular malformations (VM) may become symptomatic and extensive, leading to deranged coagulation and bleeding. Sirolimus®, an antiangiogenic agent, has recently emerged as treatment for VM. We report its short-term outcomes for VMs of the extremities. Case 1: A 47-year-old female reported left forearm pain. MRI confirmed a VM. Lesion shrinkage and pain relief were not achieved despite sclerotherapy. After 5 months on Sirolimus®, improved pain, decreased forearm circumference and decreased lesion size on MRI were observed. Case 2: 62-year-old male reported left knee pain. MRI and biopsy confirmed a VM. After 3 months on Sirolimus®, improved pain, decreased leg circumference, and decreased lesion size on MRI were observed. Our series demonstrates that Sirolimus® is efficacious in downsizing VMs with resultant symptom relief in the short-term. Other series has likewise shown effectivity in extensive and refractory lesions, with benefits outweighing side effects.
Introduction
Vascular anomalies (VA) are categorized into malformations or tumors. Vascular malformations are developmental anomalies of vessels characterized by structural issues and errors of morphogenesis [ ]. They are divided into arterial, capillary, venous, arteriovenous, lymphatic, or combined [ ]. This is differentiated by vascular tumors by proliferative endothelial cells and aberrant vessel architecture [ ].
Many venous malformations (VM) may become aggressive and infiltrative and produce pain and deformities which warrant interventions and multidisciplinary management [ , ]. Treatment should be guided by type of anomaly, size, and how extensive the lesion is [ ].
Sirolimus®, also known as rapamycin, is an allosteric inhibitor of mammalian target of rapamycin (mTOR) initially used as an immunosuppressant to prevent organ rejection [ ]. Understanding signal transduction defects has led to the recent emergence of Sirolimus® in 2010 as a medication for VM [ ]. Current studies are investigating its efficacy and safety especially in cases refractory to standard treatments. We aim to report the short-term outcomes of Sirolimus® in patients with unresectable and refractory VMs of the extremities.
Case report (summarized Table 1)
This is a case series of 2 patients with VM deemed unresectable or refractory, and not indicated for surgery, sclerotherapy, or ablation. Following disclosure of the mechanism of action, benefits, side effects, and complications, Sirolimus® was administered once patients’ consents were granted. Institutional review board approval was obtained prior to conducting this study.
Sirolimus® was initiated with a dose of 2 mg/day. A medication diary was provided for daily documentation of vital signs and side effects. Periodic serum levels were tested, and medication dosage was adjusted to maintain trough concentration of 5-15 ng/mL.
Regular follow-up for monitoring and repeat magnetic resonance imaging (MRI) for surveillance of lesions were instructed. Decrease in the size of the lesions, improvement in signs and symptoms, and/or improvement in the function of patients were considered a positive response to treatment.
Case 1
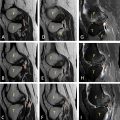
A 47-year-old, right-handed female nurse complained of intermittent swelling and pain of her left forearm since childhood, with no relief despite intake of analgesics. Physical examination revealed a soft, tender, ill-defined mass across the volar aspect of her left forearm. Grip was weak due to pain. Radiographs revealed phleboliths while MRI showed T2 hyperintense signals within the muscles of the flexor compartment ( Fig. 1 ). A vascular malformation was suspected. During this time, the patient opted to observe the lesion instead.

After 3 years, repeat MRI showed increase in the size of the lesion, associated with increasing severity of pain. Doppler sonography revealed a low-flow venous malformation with no arterial component. Sclerotherapy with 3% polidocanol was performed, however symptoms ensued. A repeat MRI after 1 year confirmed no volume change in the lesion.
Patient was hence started on Sirolimus®, with resultant improvement of visual analog scale (VAS) of pain from 8 to 3 at 3 months follow-up. Similarly, forearm circumference decreased from 28 to 23.5 cm. There were no reported side effects. An MRI 3 months ( Fig. 2 ) after starting Sirolimus® showed significant decrease in lesion size. She is currently 5 months on regimen, able to resume her work, and do activities of daily living (ADLs) with minimal episodes of pain ( Table 1 ).

| Case | Location | Symptom | Previous treatment | Sirolimus dose | Serum level (ng/dL) 2w 4w | VAS 0 1m 3m | COL (cm) 0 1m 3m | Max tumor area (MRI, mm 2 ) 0 3m | Adverse effects | F/U |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Flexor muscles in the forearm | Pain Motor weakness | Sclero- therapy | 2 mg/d | 13.3 14.7 | 8 5 3 | 28 24 23 (−18%) | 1299 931 (−29%) | None | 5 m |
| 2 | Anterior compartment in the leg | Pain | None | 2 mg/d | 13.5 16.4 | 5 4 3 | 37 35 33 (−11%) | 837 704 (−16%) | pruritic rash on the trunk | 4 m |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree