Abstract
This chapter includes thyroid anatomy, physiology, and radiopharmaceuticals used for nuclear medicine imaging. There are normal images, as well as many images and text for common thyroid conditions. The chapter also contains issues related to the radioiodine therapy of both benign conditions and thyroid cancer. Parathyroid and salivary gland imaging is presented, as well.
Keywords
thyroid uptake, thyroid scan, hyperthyroidism, thyroid cancer, thyroid nodules, radioiodine therapy, parathyroid imaging, salivary gland imaging
Chapter Outline
Thyroid Radioiodine Uptake and Imaging
Iodine-131 Therapy in Benign Thyroid Disease
Iodine-131 Therapy in Hyperthyroidism
Patient Preparation and Post-Treatment Expectations for Iodine-131 Hyperthyroid Therapy
Iodine-131 Therapy in Thyroid Cancer
Patient Preparation for Iodine-131 Cancer Therapy
Early Side Effects and Late Sequelae of Iodine-131 Therapy
Parathyroid Imaging and Localization
Thyroid Radioiodine Uptake and Imaging
The use of iodine-131 ( 131 I) for measuring thyroid functional parameters and imaging the gland has historically served as a nucleus in the evolution of the field of nuclear medicine, as well as molecular imaging. Although significant changes have taken place in the radionuclide approach to thyroidology, many essential principles remain unchanged. Therefore, a basic understanding of these principles is necessary before interpretation of the functional or imaging data should be attempted.
Most thyroid imaging techniques capitalize on some phase of hormone synthesis within the thyroid gland. Iodides or iodide analogs are actively transported by the glycoprotein sodium iodide symporter (NIS) into the thyroid follicular cell, a process referred to as trapping, as the first step in thyroid hormone synthesis. The iodides are then oxidized by thyroid peroxidase to iodinium (I + ) and bound to tyrosyl moieties, a process called organification , to form mono- and di-iodinated tyrosine (MIT and DIT). These are then coupled to form tri-iodothyronine (T 3 ) and thyroxine (T 4 ). It is an important distinction that the iodide analogue, sodium technetium-99m ( 99m Tc) pertechnetate (Na + TcO 4 − ), is trapped, but does not undergo organification to form thyroid hormone; instead, after trapping, it slowly “washes” out of the gland.
Radiopharmaceuticals
The radioactive iodine ( 123 I sodium) and technetium ( 99m Tc-pertechnetate) constitute the radionuclides used in imaging the thyroid gland. Both 123 I and 131 I are used for iodine uptake tests. Only 131 I is commonly used for thyroid therapy.
Iodine-131
Iodine-131 decays by beta emission and has a half-life of 8.04 days. The principal gamma emission of 364 keV is considerably higher than the ideal for imaging with gamma cameras. A ![]() -inch-thick (1.3 cm) sodium iodide crystal has only 30% efficiency for these photons.
-inch-thick (1.3 cm) sodium iodide crystal has only 30% efficiency for these photons.
Major advantages of 131 I include its low price and ready availability. Its long physical half-life and abundant beta emission, which cause a relatively high radiation dose to be delivered to the thyroid with a relatively low whole-body dose, make 131 I an ideal radiotherapeutic agent for treating certain thyroid disorders. However, these properties also render it unsuitable for routine imaging of the thyroid gland. Its long half-life is also of advantage in whole-body scanning for the detection of functioning thyroid cancer metastases because imaging can be done over several days to allow for optimum concentration by the metastatic lesions, resulting in higher tumor-to-background ratios and improvement of imaging sensitivity.
Iodine-123
Iodine-123 has excellent physical properties for imaging the thyroid gland. Like 131 I, its biochemical behavior is identical to that of stable iodine. Iodine-123 decays by electron capture, with a photon energy of 159 keV and a half-life of 13 hours. The gamma emission of 123 I allows excellent imaging (≈80% efficiency for a ![]() -inch-thick crystal) with low background activity. It provides considerably lower doses of radiation to the thyroid with comparable activity than does 131 I. Iodine-123 is the iodide of choice for thyroid imaging.
-inch-thick crystal) with low background activity. It provides considerably lower doses of radiation to the thyroid with comparable activity than does 131 I. Iodine-123 is the iodide of choice for thyroid imaging.
Technetium-99m
Technetium-99m pertechnetate is trapped by the thyroid in the same manner as iodide but is not organified; therefore, it is released over time as an unaltered pertechnetate ( 99m TcO 4 − ) ion. Its short physical half-life of 6 hours and principal gamma energy of 140 keV are ideal for gamma camera imaging (greater than 90% efficiency with a ![]() -inch-thick crystal). These physical characteristics and its ready availability are distinct advantages for thyroid scanning. In addition, the low absorbed dose to the thyroid permits administration of higher doses, allowing for more rapid imaging of the gland with minimal motion artifact. Only 1% to 5% of administered 99m Tc-pertechnetate is normally trapped by the thyroid, so image background levels are higher than those with radioiodine. On a 99m Tc-pertechnetate scan, the salivary glands are usually well seen in addition to the thyroid. As a result, unless a patient has increased thyroid uptake of 99m Tc-pertechnetate as in diffuse toxic goiter (Graves disease), the scan can usually be distinguished from an 123 I scan by excellent visualization of the salivary glands. Technetium-99m pertechnetate is preferred over radioiodine when a patient has recently received thyroid-blocking agents (such as iodinated contrast agents), is unable to take oral 123 I, or when the study must be completed in less than 2 hours.
-inch-thick crystal). These physical characteristics and its ready availability are distinct advantages for thyroid scanning. In addition, the low absorbed dose to the thyroid permits administration of higher doses, allowing for more rapid imaging of the gland with minimal motion artifact. Only 1% to 5% of administered 99m Tc-pertechnetate is normally trapped by the thyroid, so image background levels are higher than those with radioiodine. On a 99m Tc-pertechnetate scan, the salivary glands are usually well seen in addition to the thyroid. As a result, unless a patient has increased thyroid uptake of 99m Tc-pertechnetate as in diffuse toxic goiter (Graves disease), the scan can usually be distinguished from an 123 I scan by excellent visualization of the salivary glands. Technetium-99m pertechnetate is preferred over radioiodine when a patient has recently received thyroid-blocking agents (such as iodinated contrast agents), is unable to take oral 123 I, or when the study must be completed in less than 2 hours.
Dosimetry
Radiation doses to the adult thyroid and whole body for the radioiodines and 99m Tc-pertechnetate are presented in Appendix E with imaging protocols. With the usual administered activities for scanning, the radiation to the thyroid gland is comparable for 123 I and 99m Tc, and the whole-body dose is only slightly greater with 99m Tc. Both agents provide considerably less radiation dose to the thyroid and to the total body than does 131 I. The dose to the thyroid from 131 I is about 1 rad/µCi (10 mGy/0.037 MBq), which is about 100 times greater than that from 123 I, which is about 1 rad/100 µCi (10 mGy/3.7 MBq) of administered activity. The absorbed thyroid dose from 99m Tc-pertechnetate is about 1 rad/5000 µCi (10 mGy/185 MBq).
Because radioiodides cross the placenta (as does 99m Tc-pertechnetate) and because the fetal thyroid begins accumulation of iodine at about the 12th week of gestation, care must be taken when administering these radiopharmaceuticals during pregnancy. They are also secreted in breast milk and may be transferred to nursing infants. Nursing can usually be resumed 12 to 24 hours after the administration of 99m Tc-pertechnetate and about 2 to 3 days after 123 I administration. Because of fetal irradiation from bladder activity in early pregnancy and the danger of fetal thyroid ablation after 12 weeks of gestation, 131 I is contraindicated during pregnancy. When 131 I is administered in any form to postpartum women, nursing should be stopped, and any pumped breast milk discarded, because the US Nuclear Regulatory Commission (NRC) recommends that nursing should be discontinued entirely if administered activities of 131 I exceed about 1 µCi (0.04 MBq).
On an administered activity basis, the dose to the thyroid is greater in infants and children than in adults, and considerably smaller scanning and uptake doses should be administered to pediatric patients (see Appendix D ). In addition, because the radiation dose to the pediatric thyroid from 131 I nears the level shown to increase the incidence of thyroid carcinoma, 131 I is not recommended for scanning children. In this context, it is worth noting that the incidence of thyroid cancer in children receiving moderate thyroid absorbed doses from 131 I is greater than with high therapeutic doses to the thyroid, which destroy significant amounts of thyroid tissue.
Although the radiation dose to the thyroid from 131 I can be estimated by knowing the administered activity and the thyroid uptake, a complex of less easily determined factors, including thyroid size, biologic half-life of iodine in the gland, size of the iodine pool, and spatial distribution of iodine in the gland, can change the absorbed dose by up to a factor of 10 in any given patient.
Radioiodine Uptake Test
The radioactive iodine (RAI) uptake test is easily performed and gives a useful clinical index of thyroid function. The main purposes of an uptake examination before radioiodine therapy are to ensure that the thyroid will take up RAI and to determine how much activity to administer as a treatment dose. The diagnosis of hyperthyroidism or hypothyroidism, however, is not made by using radioactive iodine uptake but should be made by serum measurements of thyroid hormone and thyroid-stimulating hormone (TSH) levels. However, the thyroid uptake can be used to differentiate hyperthyroidism caused by Graves disease, with elevated uptakes from subacute thyroiditis or factitious hyperthyroidism, in which the uptakes are typically very low ( Table 4.1 ).
| Radioiodine Uptake | |
| Differentiating hyperthyroidism from other causes of thyrotoxicosis | High uptake: Graves disease; toxic nodular goiter Low uptake: subacute thyroiditis; factitious thyroiditis |
| Determine 131 I dosage for treating hyperthyroidism or postsurgical ablation of residual thyroid tissue | For calculating patient-specific doses or refining empiric doses |
| Thyroid Scan | |
| Hyperthyroidism | To determine etiology: Graves disease (diffuse toxic goiter), multinodular toxic goiter; toxic adenoma; subacute thyroiditis |
| Thyroid nodules | To determine functional status when appropriate, including inconclusive biopsy or ultrasound results |
| Evaluate inconclusive or confounding laboratory tests suggestive of thyroid disease | To determine a possible thyroid gland etiology |
| Mediastinal or thoracic inlet/cervical mass | Confirm possible substernal goiter |
| Ectopic thyroid tissue presenting as mass | Cervical mass along track of thyroglossal duct; lingual thyroid |
| Post-thyroidectomy estimation of residual thyroid tissue | Determine need or dose of ablative therapy |
| Congenital thyroid abnormalities | Hypothyroidism in infants, including thyroidal defects in organification |
Principle and Technique
Thyroid uptake is based on the principle that the orally administered radiopharmaceutical is concentrated by the thyroid gland in a manner that reflects the gland’s handling of stable dietary iodine and thus the functional status of the gland. The higher the uptake of the radiopharmaceutical, the more active the thyroid; conversely, the lower the uptake, the less functional the gland. Uptake is expressed as the percentage of the administered activity in the thyroid gland at a given time after administration (usually at 4 to 6 hours and 24 hours). For radioiodine uptakes, the normal range for both children and adults is about 10% to 30% for 24-hour uptake determinations. The normal range for a 4- to 6-hour uptake is about 5% to 15%.
To aid absorption, it is advisable that patients receive nothing by mouth (NPO) at least 2 to 4 hours before administration; ideally, NPO status should begin at midnight the day before oral administration of the radionuclide. It is also helpful to determine the functional status of the gastrointestinal tract before administering the radiopharmaceutical because vomiting or diarrhea may hinder adequate absorption.
To begin the test, about 5 to 10 µCi (0.2 to 0.4 MBq) of 131 I-sodium or 50 to 100 µCi (2.0 to 4.0 MBq) of 123 I-sodium in capsule form is administered. Iodine-123 uptakes may also be performed in conjunction with an 123 I thyroid scan using a larger scanning dose of 200 to 400 µCi (7.4 to 14.8 MBq). Prior to administration to the patient, the capsule is placed in a neck phantom, and the activity is measured in counts per minute using a single-crystal nonimaging counting probe with a flat-field collimator. This measurement is later compared with counts measured in the patient’s thyroid at 4 and 24 hours to obtain the uptake percentages.
The distance from the face of the probe crystal to the anterior aspect of the patient’s neck (about 25 to 30 cm) and the method of counting are identical to those used to measure the administered capsule in the neck phantom. Correction for patient soft-tissue background activity included with the thyroid counts is made by measuring and subtracting the activity in the patient’s thigh. The number of counts obtained may then be subtracted from the neck reading to estimate counts isolated in the thyroid gland. Correction of capsule-in-phantom counts using room background and for interval radioiodine decay at 4 and 12 hours is also performed.
All measurements are usually performed twice, for 1 to 2 minutes each, and are then averaged to calculate the percentage uptake, using the following formula:
% thyroid uptake = neck counts − thigh counts / corrected capsule counts × 100 %
A sample technical protocol for performing radioiodide uptakes can be found in Appendix E .
Factors Affecting Iodine Uptake
Increased blood pool iodides compete with administered radioiodines for trapping and organification by the thyroid gland. An increase in the body iodide pool is most frequently caused by increased dietary intake, which may significantly reduce the uptake values obtained. Conversely, a decrease in ambient iodine produces an “iodide-starved gland,” which may trap and bind greater amounts of radioactive iodide, producing elevated uptake values. In addition, geographic differences in dietary iodide intake give rise to local variations in the normal range. These factors, along with differences in technical aspects of the procedure among laboratories, make it advisable for each facility to determine its own range of normal values.
Good renal function is essential to normal radioiodine uptake. In patients with chronic renal failure, iodides usually excreted by the kidneys are retained, producing an increase in the stable iodide pool. This dilutes the percentage of radioiodine taken up by the gland, resulting in low uptake determinations. Large meals shortly before or after oral administration of radioiodine can slow or decrease absorption and interfere with uptake measurements.
Numerous medications and iodinated contrast agents also affect radioiodine uptake. To perform a radioiodine uptake successfully, these medications must be withheld for appropriate periods before the uptake procedure is attempted ( Table 4.2 ). Also, careful interviewing of patients undergoing the uptake test is necessary to determine whether there is a history of antithyroid drugs, thyroid hormones, iodide preparations, or iodinated contrast agents used in computed tomography, radiographic, and angiographic procedures. Notably, β-blockers such as propranolol, which are commonly used to combat the clinical manifestations of hyperthyroidism, do not affect the function of the gland and therefore do not interfere with thyroid uptake of the radioactive iodine.
| Medication | Withdrawal Time a |
|---|---|
| Carbimazole | 3 days |
| Bromides | 1 week |
| Corticosteroids | |
| Methimazole (Tapazole) | |
| Propylthiouracil | |
| Multivitamins b | |
| Nitrates | |
| Perchlorate | |
| Salicylates (large doses) | |
| Sulfonamides | |
| Thiocyanate | |
| Iodine solution (Lugol or SSKI) b | 2–3 weeks |
| Iodine-containing antiseptics b | |
| Kelp b | |
| Some cough medicines and vitamin preparations | |
| Tri-iodothyronine (Cytomel) | 3–4 weeks |
| Thyroid extract (Synthroid, Proloid) | |
| Intravenous contrast agents (water soluble) | 1–2 months |
| Amiodarone | 3–6 months |
a Time that patient should wait after medication is discontinued in order to obtain an accurate uptake.
b These relate to hyperthyroid patients. For hypothyroid patients, a 6-week interval is recommended.
Elevated Radioiodine Uptake
Primary hyperthyroidism caused by diffuse toxic goiter (Graves disease) typically produces clearly elevated iodine uptakes. On the other hand, hyperthyroidism produced by toxic nodular goiters (Plummer disease) may yield uptake values in the high, normal, or mildly elevated range. Therefore, a normal or borderline elevated radioiodine uptake alone cannot be used to exclude the diagnosis of hyperthyroidism when it is clinically suspected.
Elevated uptakes also may be produced by a variety of other conditions ( Box 4.1 ). The so-called “iodine rebound” phenomenon may result from the release of TSH by the pituitary after sudden withdrawal from thyroid hormone suppression therapy. It may also result from hormone synthesis rebound after withdrawal of antithyroid drugs, such as propylthiouracil. High uptakes can also be the result of abnormally increased production of TSH by the pituitary or secretion of TSH-like hormones by gonadal or chorionic tumors (secondary hyperthyroidism), or may occur in the recovery phase of subacute thyroiditis.
Increased Uptake
Hyperthyroidism (diffuse or nodular goiter)
Early Hashimoto thyroiditis
Recovery from subacute thyroiditis
Rebound after abrupt withdrawal of antithyroid medication
Enzyme defects
Iodine deficiency or starvation
Hypoalbuminemia
Thyroid-stimulating hormone
Tumor-secreted stimulators (gonadal and chorionic origin)
Pregnancy
Decreased Uptake
Hypothyroidism (primary or secondary)
Iodine overload (especially radiographic contrast)
Medications (see Table 4.1 )
Subacute or autoimmune thyroiditis
Thyroid hormone therapy
Ectopic secretion of thyroid hormone from tumors
Renal failure
Reduced Radioiodine Uptake
Primary or secondary hypothyroidism may produce decreased radioiodine uptake. Primary hypothyroidism is a failure of the gland to respond to TSH, whereas secondary hypothyroidism is caused by insufficient pituitary secretion of TSH. Serum TSH and thyroid hormone assays are necessary to establish the diagnosis.
As mentioned, a number of medications may cause decreased radioiodine uptake ( Table 4.2 ). Withdrawal of the medications for the times given in the table is necessary to assure accurate measures of thyroid function. Rarely, well-differentiated thyroid cancers, teratomas, and struma ovarii can be sites of ectopic secretion of thyroid hormone, which may suppress thyroidal uptake.
Thyroid Gland Imaging
Iodine-123 sodium and 99m Tc-pertechnetate remain the radiopharmaceuticals of choice for routine imaging of the thyroid gland. Both provide images of excellent quality, although the higher-energy photons of 131 I may be preferable for imaging deep ectopic tissue when necessary. Although 123 I is more expensive than 99m Tc, it has the advantage of being able to provide concurrent radioiodine uptake and images with a relatively low radiation dose. In addition, 123 I images reflect both trapping and organification in the gland.
The indications for scintigraphic thyroid imaging include:
- •
To differentiate among the causes of hyperthyroidism, notably Graves disease from toxic nodular goiter. This distinction is important in determining therapeutic radioiodine dose.
- •
To differentiate between hyperthyroidism and other causes of thyrotoxicosis (e.g., Graves disease from subacute, silent, or postpartum thyroiditis or factitious hyperthyroidism). In the latter entities, there are symptoms of hyperthyroidism with elevated serum levels of thyroid hormone, but radioiodine studies reveal that the radioiodine uptake in the gland is low and visualization is poor.
- •
To determine the functional status of a thyroid nodule.
- •
To determine whether a cervical or mediastinal mass is thyroid tissue.
- •
To locate ectopic tissue, such as a lingual thyroid.
- •
To assist in the evaluation of congenital hypothyroidism or organification defects.
Even with a history of recent radioiodinated contrast or suppressive medication administration, it is frequently possible to obtain reasonably good anatomic assessment of the thyroid gland with 99m Tc-pertechnetate when it is not possible with radioiodine. This is, in part, related to the significantly greater administered activity used for pertechnetate imaging than for radioiodine. When feasible, however, the patient should return after an appropriate interval of withdrawal from the interfering drug for a more definitive examination.
Technical Imaging Protocol
Iodine-123
Imaging with sodium 123 I may be performed after the oral administration of 200 to 400 µCi (7.4 to 14.8 MBq) to a fasting patient. Imaging is usually performed 4 hours later but may be done at 24 hours, although a longer acquisition time is required. Images of high quality are obtained using a 100,000-count or a 7- to 10-minute acquisition with a pinhole collimator. Images are obtained in anterior and bilateral oblique camera positions with the patient remaining in a fixed supine position with neck extended. The oblique images are essential for the identification of laterally and posteriorly placed nodules that might be missed with simple anterior imaging. The position of a palpable nodule under investigation should be documented with a view obtained with a 99m Tc or 57 Co marker placed on the lesion. This aids in an accurate correlation of the physical and scan findings. An additional anterior image with a marker on the sternal notch aids in locating the position of the thyroid with respect to the mediastinum and thoracic inlet.
Complete technical protocols for performing thyroid scintigraphy using 99m Tc-pertechnetate and 123 I can be found in Appendix E .
Technetium-99m Pertechnetate
The thyroid is imaged 5 to 30 minutes after the intravenous administration of 2 to 10 mCi (74 to 370 MBq) of 99m Tc-pertechnetate, using a scintillation camera with a pinhole collimator. Anterior and left and right anterior oblique images are then obtained for 100,000 to 250,000 counts (or 5 minutes) each, with the patient supine and neck extended. The procedure is otherwise similar to that using 123 I.
Normal Images
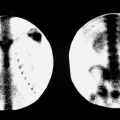
The normal thyroid gland is a bilobed organ with reasonably homogeneous distribution of activity in both lobes ( Fig. 4.1 ). In adults, the entire gland weighs between 15 and 20 g, and each lobe measures about 2 × 5 cm. Slight asymmetry in the sizes of the lobes is common, with the right lobe generally dominating. The lobes are usually joined inferiorly and medially by the thyroid isthmus, which may demonstrate relatively decreased activity compared with the adjacent lobes. In some instances, complete absence of activity is noted in this region. In a small number of patients, a pyramidal lobe (thyroglossal duct remnant containing functioning thyroid tissue) arises from the isthmus or medial aspect of one lobe and extends superiorly and medially. Although this is a common variation, it may be accentuated in partial thyroidectomy patients and in patients with diffuse thyroid abnormalities such as Graves disease or Hashimoto thyroiditis. Less common variants of thyroid configuration include congenital absence of one lobe, substernal extension of the gland, or a sublingual thyroid with functioning tissue at the base of the tongue.

The most common artifact of 99m Tc-pertechnetate studies of the thyroid is produced by activity secreted by the salivary glands and swallowed by the patient. This usually presents as a linear area of esophageal activity in the midline of the image. If this complicates interpretation or causes confusion with a pyramidal lobe, additional imaging should be performed using oblique images or after clearing the esophagus by having the patient drink water.
Clinical Applications: Nodular and Other Benign Diseases
Thyroid Nodules
Thyroid nodules are common. In North America, palpable nodules occur in 4% to 7% of adults and more than 30% have nodules on ultrasound. Thyroid cancer occurs in 5% to 15% of nodules. Iodine-123 is the radiopharmaceutical of choice for imaging thyroid nodules, as 99m Tc can be unreliable in some settings. Conventionally, nodules are classified on radionuclide scans with respect to the amount of activity present relative to activity in the remainder of the normal thyroid gland. Cold nodules (85% to 90% of all nodules) demonstrate an essential absence of activity, whereas hot nodules (5% of nodules) are identified by focally increased activity. About 10% of nodules are neither hot nor cold but contain activity comparable to that of the surrounding gland and are frequently termed “warm” nodules.
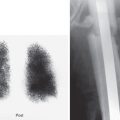
Although thyroid ultrasound and fine-needle aspiration (FNA) biopsy have essentially supplanted radionuclide imaging as the initial investigative procedure for palpable thyroid nodules, imaging can be useful in selected patients ( Fig. 4.2 ). Nodules with suppressed TSH levels may indicate that a nodule is producing high levels of thyroid hormone and a thyroid scan can determine whether it is consistent with an autonomous hyperfunctioning (“hot”) nodule. For a nodule with indeterminate FNA cytology results (15%), if the nodule is seen to be nonfunctioning on thyroid scan, this may boost the impression of a suspicious nodule that needs to be observed closely or removed. This may occur when a benign follicular adenoma cannot be histologically distinguished from a follicular malignancy.

Cold Nodule
A nonfunctioning thyroid nodule ( Fig. 4.3 ) is essentially a nonspecific finding and may be the result of any of numerous pathologies ( Table 4.3 ), the most common of which is a colloid cyst. Although 85% of cold nodules are benign, the fact that a small percentage prove to be cancerous is sufficient to warrant further assessment with sonography, including a survey of cervical lymph nodes, depending on the clinical circumstances. If the nodule has suspicious or equivocal sonographic features, FNA should be performed. The reported percentage of solitary cold nodules harboring thyroid cancer varies, depending on the clinical bias of the particular study but is generally thought to be about 15% to 20%. The likelihood of carcinoma significantly increases if the patient is young. Suspicion is further increased if associated lymphadenopathy is identified. If a history of previous head and neck radiation therapy is elicited, a cold nodule has about a 40% chance of being malignant ( Box 4.2 ).