Tracheobronchial Stents
K. Pallav Kolli
Roy L. Gordon
Tracheobronchial stenosis can be treated by surgical resection and reanastomosis, balloon dilation, laser therapy, or stenting (1,2,3). Surgical treatment of stenoses with end-to-end reanastomosis is only possible when there is sufficient length of airway available and when the patient is in physical condition to survive repeat surgery. Although endobronchial mechanical debulking and ablative techniques can successfully treat small protrusions within the airway, they cannot effectively deal with circumferential lesions with long scarred segments, with stenoses caused by extrinsic airway compression, with functional airway collapse, nor with fistulae or air leaks. Expandable stents can be used in all these conditions.
Tubular silicone stents have been used to stent tracheobronchial stenosis; however, they have a thick wall that reduces the cross-sectional diameter of the airway, are prone to migrate, and interfere with mucociliary mechanisms leading to plugging of the stent with inspissated secretions. Expandable, bare metallic stents are thinwalled and become incorporated into the bronchial wall or epithelialized, preventing migration and possibly permitting ciliary activity to continue. Expandable metallic stents have other advantages. They offer a rapid and effective means for opening narrowed airways, resulting in excellent relief of symptoms. Stents are well tolerated by patients who become unaware of their presence shortly after placement. Expandable stents are delivered in their nonexpanded state so that their delivery systems are of small caliber. They are generally flexible over-the-wire systems allowing placement into second-order branches of the bronchial tree as well as into the trachea and mainstem bronchi. Expandable stents can be placed using a flexible rather than a rigid bronchoscope. They can even be placed over a guidewire without bronchoscopy.
This chapter focuses on expandable metallic stents that are often placed by interventional radiologists in patients with benign strictures. Modifications of these techniques, in particular stent selection, may be necessary when treating malignant obstructions with large endoluminal components or airway fistulae/leaks.
Indications
1. Expandable stents have been successfully used in both benign and malignant conditions in severely symptomatic patients who have not been amenable to other forms of treatment (1,2). The decision to treat is heavily based on the degree of the patient’s symptoms. These stenoses may be (a) endoluminal, (b) in the wall of the airway itself (intramural), or (c) a result of external compression.
a. Benign obstruction: post-lung transplantation anastomotic strictures, tracheal strictures following prolonged intubation, postinfection inflammatory strictures, tracheomalacia, relapsing polychondritis, Wegener granulomatosis, AIDS, external compression from benign mediastinal masses or fibrosis, and airway collapse related to chronic obstructive pulmonary disease
b. Malignant obstruction : Malignant airway obstructions may arise from primary or metastatic tumors or invaded lymph nodes. Stenting has been used for symptomatic palliation, regardless of tumor type, provided a patent airway distal to the obstructed segment is present. Malignant obstructions associated with prolonged (>2 weeks) lung collapse should be approached with caution because treatment may not result in improvement and risks the release of infected secretions into other portions of the lungs.
Contraindications
1. High tracheal lesions where placement would result in the upper end of the stent being in the vocal cords
2. Presence of active inflammation of the airways
3. Absence of a patent distal landing site
Preprocedure Preparation
1. Pulmonary function tests: These are helpful in evaluating patients’ subjective symptoms and allowing objective evaluation of pre- and posttreatment status (6).
2. Thin-slice computed tomography (CT): This study should be performed using a carefully developed protocol in both inspiration and expiration and during forced vital capacity at the previously identified site of maximal narrowing. The CT scan yields important three-dimensional airway anatomy for characterization of the lesion and stent selection. Multiplanar reconstructions are especially helpful in assessing the extent of airway abnormality and obtaining airway measurements. The dynamic changes can highlight airway collapse from malacia as well as focal or diffuse air trapping distal to the narrowed segment.
3. Bronchoscopy in the conscious, freely breathing patient: Bronchoscopy performed at the time of stent placement may underestimate the degree of functional narrowing because the patient is typically under general anesthesia with positive pressure respiration.
4. Treatment of active inflammation
5. Coagulation studies
6. Review of previous studies
7. Preliminary selection of stent type, diameter, and length: No stent is ideal nor universally accepted for use in the tracheobronchial tree. The ideal stent should be available in suitable lengths and diameters; be easy to see fluoroscopically; be easy to place accurately; be resistant to migration, fracture, and permanent deformation; should not block ciliary action or mucus clearance; should be easily removable; and should be well tolerated. No single stent or stent category fulfills each of these criteria. Covered self-expanding metallic stents are generally reserved for situations in which an air leak is present, although some operators favor them because they are less difficult to remove than bare self-expanding metal stents and are better able to prevent tumor or granulation tissue ingrowth.
8. Coordination of other involved medical services including an anesthesiologist who is typically responsible for the periprocedural medical management of the patient as well as intraprocedural anesthesia and a pulmonologist who performs bronchoscopy.
Anatomy
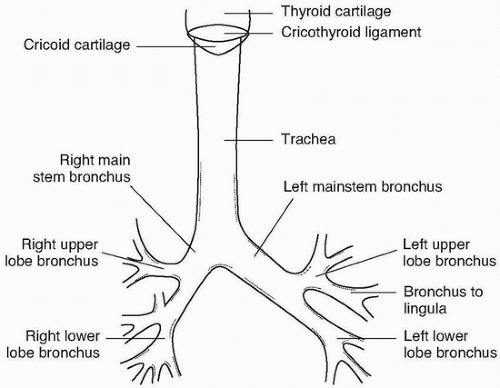
The important tracheobronchial anatomy is shown in Figure e-78.1.
1. In general, the structures are larger in men than in women, and there is some variation from patient to patient. With increasing age, there is mild enlargement in tracheobronchial dimensions.
2. The trachea is about 110 to 120 mm in length, extending from the larynx at about C6 to the carina at T5. Typical diameters for man and woman tracheas, respectively, are coronal, 19.5 and 16.5 mm, and sagittal, 20.5 and 17.0 mm.
3. The right main stem bronchus is about 25 mm in length, about half the length of the left, and about 15 mm in diameter.
4. The left main stem bronchus is about 50 mm long and 13 mm in diameter.
5. Major branch airways have diameters in the 6- to 12-mm range.
Procedure
1. The procedure is performed on a fluoroscopy table, usually under general anesthesia, using an endotracheal tube of 8.0- to 8.5-mm diameter. This allows optimal airway management and a controlled atmosphere that is comfortable and safe for both the patient and the operators. Accurate stent placement is best achieved under these conditions. Alternatively, the airway can be managed with a laryngeal mask airway (LMA), which is an advantage when stenting in the trachea. Other operators use conscious sedation.
2. The bronchoscope is passed via a right-angled connector coaxially down the endotracheal tube. This arrangement provides an air seal and allows simultaneous bronchoscopy and ventilation. A small adult-sized scope is usually chosen. A large adult scope provides better vision and a large instrument channel if biopsy is required but is less conveniently used through the smaller endotracheal tubes.
3. Bronchoscopy is performed to visualize the nature and extent of the lesions. Lesions seen at this time, in a patient who is under general anesthesia and is being ventilated, may differ from and appear less serious compared with lesions seen during spontaneous respiration in the normal state. Lesion length can be measured by moving the bronchoscope in and out from one margin of the lesion to the other. These findings are correlated with the preprocedure CT.