Abstract
Objective
To develop a treatment that enhances recovery from envenomation-induced lesions caused by Bothrops jararaca venom by using ultrasound in combination with gold nanoparticles (GNPs).
Methods
A total of 108 Swiss mice were arranged into nine groups. The animals underwent necrotic induction with 250 µg B. jararaca venom (BjV) and were treated with ultrasound (U) at 1 MHz frequency at an intensity of 0.8 W/cm² for 5 min, 30 mg/L GNPs, and anti-bothropic serum (AS) in the following combinations: saline solution (SS); BjV; BjV + AS; BjV + AS + U; BjV + GNPs + AS; BjV + GNPs + AS + U; BjV + GNPs; BjV + GNPs + U; and BjV + U. The necrotic area, histology, oxidative stress, oxidative damage, and anti-oxidant system were assessed to evaluate the effects of the treatments.
Results
Treatments that included GNPs, U, and/or AS demonstrated reductions in necrotic area, increases in angiogenesis and fibroblast means, decreases in inflammatory infiltrates, and improvements in collagen synthesis. Additionally, there was an increase in oxidants and oxidant damage within the gastrocnemius muscle, along with an increase in anti-oxidants. Furthermore, systemic effects appear to have been achieved, improving the anti-oxidant system at the cardiovascular and renal levels.
Conclusion
The use of GNPs and U may be effective at treating lesions caused by B. jararaca snake venom.
Background
Accidents caused by venomous animals are considered to be a neglected public health problem due to the scope and severity of these incidents [ ]. Currently, 4.5–5.4 million accidents occur as a result of contact with snakes annually [ ]. In Brazil, there have been at least 20,000 accidents related to Bothrops snakes [ ]. Bothrops jararaca venom (BjV) causes serious local and systemic consequences such as inflammation and tissue damage, hemostatic disorders, hemorrhage, edema, myonecrosis [ ], and pain [ ]. Systemic complications include shock and acute renal failure, both with multifactorial pathogenesis [ ].
When a muscle injury occurs, a series of immune responses are initiated to eliminate damaged tissues as well as possible infectious agents involved, leading to tissue destruction and reconstruction. This host defense reaction is natural, but when uncontrolled it can cause tissue damage. Five inter-related phases have been described based on cellular and molecular events: necrotic degeneration, inflammation, regeneration, maturation/remodeling, and functional recovery [ ].
Envenomation treatment recommended by the World Health Organization includes the administration of anti-bothropic serum (AS) intravenously [ ]. This serum neutralizes the circulating venom, preventing the development of systemic effects [ ]; however, administering the serum 6 h after the bite does not prevent increases in the severity of envenomation [ ]. Thus, it is necessary to find a solution that promotes better recovery of lesions caused by envenomation.
Gold nanoparticles (GNPs) have anti-bacterial and anti-inflammatory properties [ ], and have been shown to reduce the production of reactive oxygen species (ROS) as well as oxidative damage in different animal models [ , , ]. Their use to treat lesions caused by envenomation has already been demonstrated to be helpful in necrosis caused by Loxosceles simillis [ ].
Low-intensity pulsed ultrasound is also considered an auxiliary resource in the treatment of different types of injuries. Its mechanism of action occurs through cavitation, stimulating drug transport to deeper tissues and increasing the permeability of plasma membranes. This resource is therefore widely used in the treatment of muscle injuries [ , ]. It has also been reported that ultrasound can stimulate reductions in wound size and angiogenesis, as well as increase the number of fibroblasts in lesions [ , ].
Thus, this study aimed to evaluate GNPs and ultrasound as supporting treatments for muscle injury caused by the venom of the snake B. jararaca .
Methods
Animals
A total of 108 male Swiss mice weighing 20 g were used and arranged into 9 groups, each containing 12 animals ( Table 1 ).
| Group (8 animals/group) | Intramuscular injection | Drug treatment | Treatment with electrophysical agent |
|---|---|---|---|
| 1 (Negative control) | Saline solution | – | – |
| 2 (Positive control) | Venom | – | – |
| 3 | Venom | Anti-bothropic serum | – |
| 4 | Venom | Anti-bothropic serum | Ultrasound |
| 5 | Venom | GNPs + anti-bothropic serum | – |
| 6 | Venom | GNPs + anti-bothropic serum | Ultrasound |
| 7 | Venom | GNPs | – |
| 8 | Venom | GNPs | Ultrasound |
| 9 | Venom | – | Ultrasound |
Venom
BjV samples used in this work were kindly provided by Carlos Chávez Olórtegui from the Institute of Biological Sciences at Universidade Federal de Minas Gerais (Belo Horizonte, Minas Gerais, Brazil). Lyophilized samples were stored at -20°C in the dark until needed.
Synthesis and characterization of 20 nm GNPs
The method described by Turkevith et al. [ ] was used with minor modifications, as determined by Vechia et al. [ ].
Necrotic lesion induction
For induction of the lesions, the method proposed by Costal-Oliveira et al. [ ] was used, with adaptations. A total of 250 µg BjV (previously determined; unpublished results) diluted in phosphate-buffered saline (PBS) was injected intramuscularly into the right gastrocnemius muscle. The control group received only PBS. A volume of 100 µL of venom solution or PBS per mouse was used. The necrotic lesion was evaluated 6 and 12 h after the injection, when the treatments started, and during treatment; the hemorrhage was measured before each treatment session and before euthanasia.
Treatment
Various treatment combinations were used in the animals to evaluate the isolated and joint action of pulsed ultrasound and GNPs on the necrotic lesion and whether they were associated with anti-bothropic serum. Bothropic anti-venom was also incorporated into the therapeutic schemes, as it is common to use this medicine in patients who have had accidents involving bothropic venom. Treatment started 12 h after injection of the venom into the gastrocnemius muscle of the animals and was performed once a day, lasting for 7 consecutive days. For the purposes of comparison and control of the experiment, a group of animals did not receive venom injections and were rather injected with PBS only (negative control), and another group did not receive any type of treatment after venom injection (positive control). All other groups received some type of treatment, as shown in Table 1 .
The animals were trichotomized and received treatment using 1 MHz ultrasound (Ibramed Equipamentos Médicos Ltda, Amparo, Brasil) at 0.8 W/cm 2 intensity for 5 min. The application was performed in a circular motion using saline gel (0.9%) as the conduction material. The GNP-treated groups received treatment through the application of 1 mL of 20 nm GNPs (30 mg/L) diluted in saline gel and applied topically onto the necrotic lesion. Irradiation was performed around and above the entire extension of the necrosis.
Evaluation of the necrotic area
Evaluation of the necrotic area was measured using Image J software, version 1.8. Measurements were performed with images taken under standardized light and positions prior to starting the treatment protocol (baseline measurements) and every day before applying the treatments to the mice until the fifth day, generating a total of five measurements per mouse.
Euthanasia
The animals were anesthetized with 4% isoflurane for peripheral blood collection, and chemical euthanasia was carried out using thiopental (50 mg/kg/mL intraperitoneally) 12 h after the last day of treatment. The gastrocnemius muscle was sectioned and distributed for histological and biochemical analysis. The heart and kidneys were collected for biochemical analyses.
Protein content
The protein content of the tissue homogenate was assayed using bovine serum albumin as a standard, according to Lowry et al. [ ].
Intracellular determination of reactive oxygen and nitric oxide species
Dichlorofluorescein
The production of hydroperoxides was determined by the intracellular formation of 2′,7′-dichlorofluorescein (DCF) from the oxidation of 2′,7′-dichlorodihydrofluorescein diacetate by ROS, according to the method described by Dong et al. [ ].
Nitric oxide formation indicator
To evaluate nitric oxide (NO) levels, the stable metabolite nitrite was measured via a Griess nitrite assay, as described by Chae et al. [ ].
Oxidative damage markers
Sulfhydryl content
To determine total thiol groups, the color reagent 5,5′-dithiobis-(2-nitrobenzoic acid) method was used, as described by Aksenov et al. [ ].
Protein carbonylation
Protein oxidation was determined by quantifying carbonyl proteins through the reaction of carbonyl groups with dinitrophenylhydrazine. The carbonyl content was determined as described by Levine et al. [ ].
Anti-oxidant defenses
Superoxide dismutase
Superoxide dismutase (SOD) was measured by inhibition of adrenaline auto-oxidation, as described by Bannister and Calabrese [ ].
Glutathione
Glutathione (GSH) levels were determined as described by Hissin and Hilf [ ], with some adaptations. GSH was measured in epithelial tissue homogenate after protein precipitation with 1 mL 10% trichloroacetic acid protein. To part of the sample 800 mM PBS, pH 7.4, and 500 µM 5,5′-dithiobis-(2-nitrobenzoic acid) was added. Absorbance was read at 412 nm after 10 min. A reduced glutathione standard curve was used to calculate GSH levels within the samples.
Histological analysis
The animals were euthanized and had the gastrocnemius muscle of the right hind paw removed and post-fixed in a 4% paraformaldehyde solution in 0.1 M PBS (pH 7.4) 7 d after induction of the necrotic lesion. The samples were prepared as described by de Oliveira et al. [ ].
Statistical analysis
Data were expressed as mean and mean standard error, and statistically analyzed by one-way analysis of variance (ANOVA), with a post hoc Bonferroni or Dunnet test. The significance level established for the statistical tests was at least p < 0.05. GraphPad Prism versions 8.0.1 and 10.3.1 software were used.
Results
GNP characterization
GNPs were synthesized successfully, with the characteristics seen in Table 2 .
| Average size (nm) | UV-Vis (nm) | Morphology | Color |
|---|---|---|---|
| 20–30 | 523 | Spheric | Purple |
Necrosis
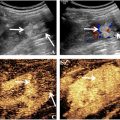
To assess the dermonecrotic lesions, photographs of necrotic areas of the dermis were taken and evaluated. Figure 1 shows the effects of all treatments on necrotic evolution. Measurement of the necrotic areas showed a decrease in necrotic area on the second and third days of treatment with AS + U ( Fig. 1 a) and on the second day of AS + GNPs + U treatment ( Fig. 1 c).

Histology
Figure 2 shows representative images of histological cross-sections of the gastrocnemius muscle stained with hematoxylin and eosin, from animals exposed to muscle injury by B. jararaca injection and treated with different combinations including saline solution (SS), BjV, AS, and ultrasound (U).


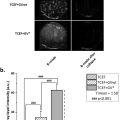
Blood vessels ( Fig. 2 a) showed an expressive increase with GNPs + U treatment. It is possible to see that the inflammatory infiltrate ( Fig. 2 b) with GNP treatment showed a significant reduction compared with the un-treated group (BjV). The mean number of fibroblasts ( Fig. 2 c) also increased with GNPs + U treatment when comparing the treated and un-treated groups. When analyzing collagen ( Fig. 2 d), the AS + GNPs, GNPs, and GNPs + U treatments were the most successful at increasing collagen production when compared with standard treatment. AS + GNPs treatment also showed a significant difference when compared with the treated and un-treated groups.
Biochemical parameters
Oxidant production
ROS, evaluated by DCF and nitrite levels, was analyzed as an oxidative parameter ( Figs. 3–5 ). The heart ( Fig. 3 ) showed a significative increase in DCF levels when comparing AS + U, AS + GNPs + U, and GNPs with standard treatment. AS treatment showed a decrease in DCF levels compared with the un-treated group. Nitrite levels showed an increase in the AS + U group when compared with the un-treated and standard treatment groups. DCF levels in the kidneys also showed increases with AS + U and AS + GNPs + U treatments compared with the un-treated and standard groups. Additionally to heart oxidant level analysis, nitrite levels within the kidneys showed an increase in the AS + U group compared with the un-treated and standard treatment groups.
DCF oxidant and nitrite levels within the gastrocnemius muscle showed an increase in the AS + U group compared with the un-treated and standard treatment groups.
Oxidative damage
Carbonyl and sulfhydryl levels within the heart showed an increase with AS + U treatment compared with the un-treated and standard treatment groups. The kidneys showed an increase in carbonyl levels with AS + U treatment compared with standard treatment. The gastrocnemius muscle showed an increase in carbonyl and sulfhydryl content levels with AS + U treatment compared with the un-treated and standard treatment groups.
Anti-oxidants
AS + U treatment within the hearts showed an increase in GSH and SOD levels when compared with the un-treated and standard treatment groups. AS + GNPs + U treatment also showed an increase when compared with standard treatment.
Within the kidneys, AS + U treatment showed an increase in GSH and SOD levels compared with the untreated and standard treatment groups. AS + GNPs + U treatment also showed an increase in SOD levels compared with the un-treated and standard treatment groups. This same treatment also showed an increase in GSH levels compared with standard treatment.
The gastrocnemius muscle showed an increase in GSH levels with AS + U and GNP treatment compared with the un-treated and standard treatment groups. SOD levels also showed an increase with AS + U treatment compared with the un-treated group.
Discussion
Low-intensity pulsed ultrasound is known to modulate the inflammatory process by changing the phenotype of inflammatory cells, reducing the expression of inflammatory cytokines and limiting the infiltration of inflammatory cells [ ]. It is hypothesized that these effects are able to reach dermal levels, stimulating tissue and reducing necrosis. Furthermore, wound recovery can be stimulated by anti-oxidants as well as by the properties of 20 nm GNPs [ , ], which are easily administered topically and appear not to have toxic effects during acute exposure, contributing to an accessible treatment in terms of cost, application, and safety.
Regarding the histological results, which analyzed the local effects of the various treatments, the GNPs + U group showed a statistically significant increase in angiogenesis compared with standard treatment ( Fig. 2 a). This result was observed by Lau et al. [ ] when treating a wound caused by an incision in rats using photobiomodulation associated with GNPs. Although GNPs have demonstrated anti-angiogenic effects, the aforementioned study reported that they also did not exhibit such effects. Furthermore, there is a relationship between GNP dosage and their anti-angiogenic effect [ ]. The association between GNPs and ultrasound promotes angiogenesis signaling benefits as ultrasound likely increases the permeability of GNPs, stimulating an angiogenic effect. This mechanism, in which ultrasound increases permeability, is called phonophoresis, and as it is transdermal is considered to be safe for the administration of various drugs [ ] and therefore does not overload the digestive and hepatic systems [ ].
Ultrasound is considered to be a minimally invasive resource and safer for promoting the permeation of active drugs through the skin [ ]. Ultrasound also promotes pain relief [ , ], an important benefit in this case, as pain is one of the consequences of bothropic envenomation [ ].
Other studies have already shown that therapeutic frequencies between 1 and 3 MHz promote an increase in the penetration of small molecules through the skin [ ]. This fact supports the result of this work, where only the GNPs + U group showed a statistical difference in angiogenesis. GNPs have the potential to reduce morphological changes as well as inflammatory and oxidative stress markers [ ]. Therefore, it could be hypothesized that permeation of GNPs through phonophoresis provides a more effective action for both treatments [ ].
When damage occurs, such as that caused by envenomation, the inflammatory process is deregulated due to the mechanical action caused by the bite as well as components of the venom, such as snake venom metalloproteinases (SVMPs) and phospholipases, which play an important role in the local inflammatory response [ ]. Thus, it is important to select a treatment that has the potential to increase tissue recovery to modulate this inflammation.
Isolated GNPs can be anti-angiogenic and, thus, helpful in controlling inflammation, especially in the acute phase [ ]. This was observed in our study ( Fig. 2 a and b), where the GNP-treated group reduced inflammatory infiltrates when compared with the group that received standard treatment.
It is known that angiogenesis modulation is important during the wound-healing process [ ], and other studies have already shown that muscle morphology during damage can be improved with GNPs [ ]. Moreover, due to their nanometric scale, GNPs are able to permeate epithelial tissue [ ], an important feature in controlling lesion progression caused by bothropic venom.
GNPs administered alone suppressed angiogenesis while reducing inflammatory infiltrates ( Fig. 2 a and b). Chronic wounds are able to remain static in the inflammatory phase, which can lead to infection and make treatment difficult [ ]. Thus, an excess anti-angiogenic effect caused by GNPs, despite appearing harmful for epithelial renewal, ensures a reduction in the inflammatory process.
A promising attribute of the treatment proposal in this study has therefore been confirmed, which suggests that the combination of GNPs with ultrasound provides a balance between angiogenic and inflammatory process effects.
The groups treated with ultrasound only did not show any statistical reduction in inflammatory infiltrate ( Fig. 2 b). This result was expected as the potential of the ultrasound, in this sense, was mainly based on its active permeation activity through phonophoresis. Therefore, the literature suggests its use in combination with other drugs to meet desired objectives [ ]. However, in this study, it was not possible to observe reductions in inflammatory infiltrate in any combination involving ultrasound associated with GNPs. It was assumed that there was a loss of contact between ultrasound transmission and tissue as the size of the ultrasound head was larger than the application area, impairing ultrasound transmission. This has also been described by Draper et al. [ ], who described the necessity of total contact between the ultrasound head and the tissue.
The GNPs + U group showed an increase in the percentage of fibroblasts when compared with the non-treated group ( Fig. 2 c). This effect could be attributed to the potential of GNPs, as well as ultrasound, to promote physiological effects that result in the stimulation of fibroblasts [ , ].
SVMPs and phospholipases are responsible for degrading some components of the basement membrane, including collagen, and their intramuscular injection could lead to myonecrosis and necrosis resulting from diffusion of the venom at the site of the bite. Hemorrhagic metalloproteinases lead to poor muscle regeneration; therefore, it is important to propose treatments that act to resolve this problem [ , , ].
Collagen deposition, stimulated by the release of anti-inflammatory cytokines, is one of the steps that mark tissue repair [ , ]. The GNPs + U combination showed an increase in the percentage of collagen compaction when compared with standard treatment ( Fig. 2 d), indicating the benefits of this protocol in healing wounds caused by B. jararaca snake venom. In addition to the phonophoretic action, which appears to have favored the permeation of GNPs, ultrasound is considered to be very effective at the tissue-repair stage due to its potential to stimulate protein synthesis [ , ].
The AS + GNPs and GNPs groups also demonstrated promise at stimulating collagen in a wound caused by bothropic envenomation when compared with standard treatment ( Fig. 2 d). GNP interactions with proteins, especially within the extracellular matrix, have been widely investigated for biomedical applications due to, for example, the ease of synthesis and the adaptability of the shape and size of GNPs as needed. The extracellular matrix is the first set of biomolecules encountered by GNPs when they enter the epithelial tissue and is where collagen, the most abundant protein in animals, is found [ , ]. The literature has reported that GNPs, due to their anti-microbial and anti-oxidant effects, assist with healing and collagen repair, corroborating the results obtained in this study [ ].
When muscle damage occurs, especially during envenomation caused by the genus Bothrops , many cellular and molecular processes are involved. The inflammatory process that occurs after tissue damage is associated with an increase in ROS and a reduction in anti-oxidants, leading to oxidative damage and impairing wound healing [ , ]. Given this fact, DCF and NO were measured to evaluate oxidant levels. The gastrocnemius muscle was analyzed to evaluate parameters related to oxidative stress to assess damage caused by the venom at the bite location.
The AS + U group showed a significant increase in carbonyl levels at the gastrocnemius muscle, one of the oxidative damage markers ( Fig. 6 a), when compared with the un-treated and standard treatment groups. In counterbalance to carbonyl levels, which showed an increase in this therapeutic combination, sulfhydryl levels also increased ( Fig. 6 b). In parallel, anti-oxidant levels also increased in the same group, in addition to increasing in the group treated with GNPs alone, when compared with the standard treatment and un-treated groups ( Fig. 9 ). According to Pisoschi et al. [ ], it could be suggested that increases in oxidants and oxidative damage stimulated the anti-oxidant system, increasing its levels to avoid oxidative stress.
ROS are essential for protecting cells, but in excess, they can lead to damage and hinder the healing process [ ]. Although damage was demonstrated by carbonyl levels, there was also an increase in sulfhydryl content. Sulfhydryl groups are very vulnerable to oxidation by ROS, which results in changes in protein function. Therefore, by being the target of this direct attack, ROS act as a versatile defense system against biochemical disturbances [ ] and their increase may reflect the protective effect of the treatment on this tissue.
Myotoxins and SVMPs present in venom are able to reach the endothelium, which affects the protective role of the cardiovascular system [ ]. The AS + U, AS + GNPs + U, and GNPs groups showed a statistical increase in oxidants within the heart when compared with standard treatment, especially in DCF levels ( Fig. 4 ). AS is administered intravenously, while ultrasound and GNP treatments are applied topically to the injured area and adjacent region. It can therefore be assumed that, due to the mechanism of action and route of administration, only AS managed to keep oxidant levels low compared with the other groups. Thus, it could be suggested that electrophysical agents and GNP treatments were unable to reduce oxidant levels within the heart due to the speed of action of the venom and the scope of the treatments, which were planned to act on epithelial and muscular levels. Therefore, in this way, it is hypothesized that this treatment does not achieve systemic effects with regard to oxidant levels.