Introduction
Venous thromboembolism is a major health problem, especially among the hospitalized, the elderly, and the patient with underlying hypercoagulable states such as cancer. Deep vein thrombosis (DVT), defined as coagulated blood or clot within a deep vein of the body, constitutes one end of the spectrum of venous thromboembolism. The other end of the spectrum, and a direct sequela of both upper and lower extremity DVT, is pulmonary embolism (PE), which can have significant morbidity and mortality if not recognized early and treated.
This chapter will review the anatomy of the lower extremity venous system in addition to the prevalence, causes, risk factors, clinical findings, complications, and diagnostic imaging of lower extremity DVT.
Prevalence, Etiology, and Risk Factors
In patients with DVT, approximately 90% of cases involve the lower extremities and approximately 10% of cases occur in the upper extremities. The overall age- and sex-adjusted annual incidence has been estimated to be 48 cases per 100,000 individuals with the incidence relatively stable across age groups for males, decreased for women under 55 years of age, and increased for women older than 60 years. It is estimated that approximately 900,000 cases of venous thromboembolism occur in the United States each year. Thrombi commonly form in the deep veins of the calf and propagate cephalad into the deep venous system of the thigh. Clots in the thigh veins can embolize to the lungs. Approximately 79% of patients with PE have evidence of DVT in their legs, and conversely up to half of patients with DVT may develop PE.
The pathophysiology of DVT was initially described by Virchow who observed that a combination of endothelial trauma, venous stasis, and hypercoagulability (Virchow triad) seemed to contribute to the development of DVT in most cases. Risk factors for DVT are summarized in Table 19.1 . Acquired factors such as recent surgery or trauma, prolonged immobilization, pregnancy, oral contraceptive use, and underlying inflammatory states are the most common risk factors. Hereditary or congenital conditions such as antithrombin deficiencies (protein C and S), factor V Leiden mutation, and antiphospholipid syndrome also predispose the patient to venous thrombosis. Additionally, demographic factors play a role, with the incidence higher in females than males, African Americans than whites, and lower in Asians and Native Americans. The incidence for DVT has also been found to increase with age.
- •
DVT and PE represent both ends of the spectrum of venous thromboembolic (VTE) disease.
- •
Risk factors can be:
- •
reversible such as immobilization, surgery, or pregnancy
- •
nonreversible due to coagulation abnormalities such as factor V Leiden mutation
- •
| Hereditary Factors | Acquired Factors |
|---|---|
| Antithrombin deficiencies (protein C and S) Factor V Leiden mutation Plasminogen deficiency | Age Malignancy (advanced) Surgery (orthopedic, neurologic) |
| Non-O blood group | Trauma |
| Elevated levels of clotting factors (II, VII, VIII, IX, X, and XI) | Immobilization Pregnancy and postpartum state |
| Elevated plasminogen activator inhibitor–1 | Obesity Oral contraceptive use |
| Hyperhomocysteinemia Antiphospholipid antibody syndrome | Hormonal replacement therapy |
| Hyperviscosity syndromes | |
| Chemotherapy | |
| Heparin-induced thrombocytopenia | |
| Myelodysplasia | |
| Polycythemia vera |
Indications
The main indication for lower extremity venous ultrasound is the evaluation for possible venous thromboembolic (VTE) disease or venous obstruction in symptomatic or high-risk asymptomatic patients. Given its availability and low cost, venous ultrasound is also used for the follow-up of patients with known DVT and for determining residual thrombus load before ending anticoagulation therapy. Other indications include evaluation of venous insufficiency, reflux and varicosities, assessment for dialysis or other venous access, and venous mapping. Lower extremity venous ultrasound is also used for postprocedural assessment of venous ablation and other interventions.
Clinical findings
DVT typically originates in the calf veins at valve sites where flow is slower and can propagate proximally into the veins of the thigh. Muscle contractions and circulating plasminogen activators are constantly counteracting the formation of DVT. From the thigh, thrombus may break off and travel to the lungs, resulting in PE.
DVT usually develops in one leg at a time. Isolated calf DVT is generally asymptomatic and rarely causes clinically important PE. DVT in the calf and popliteal veins can present with unilateral calf edema, which is the most specific symptom. Pain, tenderness (often focal), redness, and increased warmth can be seen in patients with DVT but are nonspecific findings. Symptoms tend to increase with walking and improve with rest. Left untreated, the swelling may extend up the leg into the thigh. Calf pain with dorsiflexion of the foot (Homan sign) may be present, but is not a reliable sign. Occasionally, a cord may be palpated; however, a palpable cord usually represents superficial thrombophlebitis because of the increase in average body mass index (BMI).
Isolated DVT in the iliofemoral region is associated with the peripartum period, pelvic masses or pelvic surgery, oral contraceptive use, and antiphosholipid antibody syndrome. Clinically, these patients present with pain in the buttocks and/or groin. Edema caused by venous obstruction is associated with pain, duskiness of the limb, and prominent superficial collateral veins (phlegmasia cerulea dolens). It can be seen in the thigh and may progress to the entire leg.
Unfortunately, as many as 50% of patients with image-documented DVT have no specific symptoms, and nearly half of patients with “classic symptoms” of pain and swelling do not have DVT. Because clinical signs and symptoms are neither sensitive nor specific for the diagnosis of DVT, understanding its risk factors and searching for alternative diagnoses in symptomatic patients is extremely important.
Wells et al. have shown that specific criteria can be used to stratify patients with suspected DVT into high-, moderate-, and low-risk probability categories on the basis of clinical findings ( Table 19.2 ). Prevalence for DVT, as confirmed by ultrasound, was found to be 85% in the high pretest probability category, 33% in the moderate group, and 5% in the low pretest probability category. Other scoring systems exist for the clinical assessment of the risk of PE (the revised Geneva score) and for the assessment of risk of recurrence of DVT or PE (Vienna prediction model) that can be reviewed in greater detail in the provided references.
| +1 Point for each: |
| Active malignancy (within 6 months or palliative) |
| Paralysis, paresis, or recent plaster immobilization of lower limb |
| Recently bedridden >3 days |
| Major surgery/trauma within past 4 weeks |
| Localized tenderness along distribution of lower extremity veins |
| Swelling of entire lower limb |
| Calf swelling >3 cm compared with asymptomatic leg |
| Pitting edema of symptomatic leg |
| Collateral superficial veins of symptomatic leg |
| −2 Points for: |
| Alternative diagnosis at least as likely as DVT |
| Summing the points to give a probability for DVT: |
| High ≥3 points |
| Intermediate 1–2 points |
| Low 0 points |
Serologic tests such as the D-dimer test can also aid in the diagnosis of DVT, especially in patients with a low pretest probability for DVT or PE. D-dimer is a breakdown product of the cross-linked fibrin blood clot. The D-dimer assay has a high sensitivity (up to 97%) as a marker for DVT or PE. However, because it can also be elevated in many other conditions such as trauma, recent surgery, hemorrhage, cancer, and sepsis, its specificity is fairly low. A positive D-dimer test therefore has a low predictive value for establishing a diagnosis of DVT, particularly in the elderly, postsurgical patients, inpatients, or patients with malignancies. A negative test, however, has a high predictive value (>97%) for excluding DVT. It is important to note that the negative predictive value (NPV) of the D-dimer assay is dependent on the patient’s pretest probability for DVT. Wells et al. showed that the NPV is close to 100% in patients with a low pretest probability, but it drops to 85% for patients with a high pretest probability for DVT. Other factors that reduce the sensitivity of the assay are small clot burdens in patients with isolated calf thrombus that result in low levels of D-dimer in the blood, and patients who present late in the course of their DVT, as D-dimer levels usually only remain elevated for about 7 days.
Surgery is a significant risk factor for the formation of DVT. DVT related to surgery occurs intraoperatively in a large proportion of patients, and about half of all postsurgical DVT incidences resolve spontaneously within 72 hours. Most postoperative deep venous thrombi are confined to the calf veins, are small in size (<1 cm), nonocclusive, and asymptomatic. The risk of progression of postoperative DVT is greatest when there is persistence of risk factors such as immobilization, or if the original clot burden is large. The type of surgery affects the risk of developing DVT and VTE, with orthopedic surgery having approximately twice the risk of general surgery. The risk of symptomatic VTE is highest within 2 weeks of surgery and remains elevated for 2 to 3 months.
- •
Clinical evaluation is only 50% accurate for the diagnosis of lower extremity DVT.
- •
Risk scores, such as the Wells score, can be used to triage patients for further venous ultrasound imaging.
- •
The D-dimer assay is very sensitive for the presence of acute DVT but may be less sensitive for calf vein DVT.
- •
A negative D-dimer test makes the presence of DVT very unlikely.
Venous Anatomy of the Lower Extremities
The relevant deep venous anatomy of the lower extremity consists of the external iliac (EIV), common femoral (CFV), profunda femoris, and femoral veins in the thigh. Posterior to the knee, in the popliteal fossa, the popliteal vein is the major deep vein. In about 20% of cases, all or part of the deep venous system in the thigh may be duplicated. There are three pairs of intermuscular deep veins in the calf: the posterior tibial (PTV), anterior tibial (ATV), and the peroneal veins (PEV) ( Fig. 19.1 ). Numerous intramuscular veins also aid in the venous drainage of the leg. The proximal portion of the great saphenous vein (GSV) at the saphenofemoral junction (SFJ) is also included in the study.
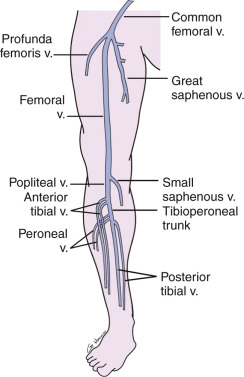
In regard to nomenclature, the superficial femoral vein is referred to as the femoral vein so it is not confused with a different superficial vein that could result in possible erroneous treatment. The terms “proximal” and “distal” should refer to the segment of the thigh or calf closer to the heart or foot, respectively; for example, the proximal femoral vein would refer to the portion of the vein closer to the hip, and the distal femoral vein would refer to the portion closer to the knee. Alternatively, the terms “cephalad” and “caudad” can be used.
The paired PTV and PEV run with their named arteries in the deep posterior compartments of the calf. The PTV arise from the plantar veins of the foot posterior to the medial malleolus and anterior to the Achilles tendon. They ascend medially in the deep compartment of the calf. The PEVs originate at the lateral aspect of the ankle and course cephalad in the central deep portion of the calf, lateral to the PTVs and medial to the fibula. The PTVs and PEVs unite in the upper calf to form the tibioperoneal trunk. The paired ATVs form from veins of the dorsum of the foot adjacent to the dorsalis pedis artery. They ascend cephalad in the anterior compartment flanking the anterior tibial artery. They course anterolateral to the tibia along the interosseus membrane. They unite with the tibioperoneal trunk to form the popliteal vein. However, anatomic variations, including duplication of the calf veins, are relatively common. The deep intramuscular veins of the calf are generally tributary branches draining into the paired deep intermuscular veins. The gastrocnemius and soleus veins are the largest deep intramuscular veins in the calf, and may or may not course next to a similarly named artery. The soleus and gastrocnemius veins are considered deep veins but are not routinely imaged. Imaging of these veins is not yet required by the Intersocietal Accreditation Commission (IAC), American Institute of Ultrasound in Medicine (AIUM), or American College of Radiology (ACR) accreditation. Nonetheless, calf DVT often originates in these intramuscular branches, which can become markedly distended with thrombus. Hence, thrombus in these intramuscular veins should be imaged and noted in the report.
The popliteal vein courses posteriorly in the popliteal fossa, superficial to the artery, into the adductor hiatus or Hunter canal in the lower thigh, and becomes the femoral vein. The soleus and gastrocnemius veins may drain into the popliteal vein or the small saphenous vein. In most patients, the small saphenous vein, a superficial vein, also drains into the popliteal vein either in the popliteal fossa or in the upper calf. The small saphenous vein is found in the subcutaneous tissues without an accompanying artery.
The femoral vein ascends cephalad from the adductor canal, posterolateral to the adjacent superficial femoral artery. In the groin, the femoral vein courses medial to the femoral artery. The profunda femoris vein parallels the profunda femoris artery, draining the posterior and lateral muscle groups of the thigh. It joins the femoral vein in the upper thigh, several centimeters below the inguinal ligament, forming the CFV. The CFV gives rise to the EIV as it crosses the inguinal ligament. In the pelvis, the EIV joins with the internal iliac vein to become the common iliac vein (CIV) before draining into the inferior vena cava (IVC).
The great saphenous vein (GSV) is a superficial vein that is found in the subcutaneous tissues of the medial calf and thigh. The GSV joins the CFV in the medial upper thigh region, near the groin, at the SFJ ( Fig. 19.2 ). The superficial inferior epigastric vein and superficial iliac circumflex vein may also be seen in this region, extending cephalad and laterally, respectively. The superficial inferior epigastric vein usually joins the GSV laterally within 1 to 2 cm of the SFJ.
- •
By convention, the proximal femoral vein refers to the vein segment closer to the groin, whereas the distal femoral vein refers to segment closer to the knee.
- •
Two deep calf veins accompany their respective artery, whereas usually only one femoral and popliteal vein accompanies its corresponding named artery. Duplications of the thigh veins can occur in about 20% of patients.
- •
Imaging of the soleal and gastrocnemius veins is not mandated by the ACR, AIUM, or IAC guidelines.
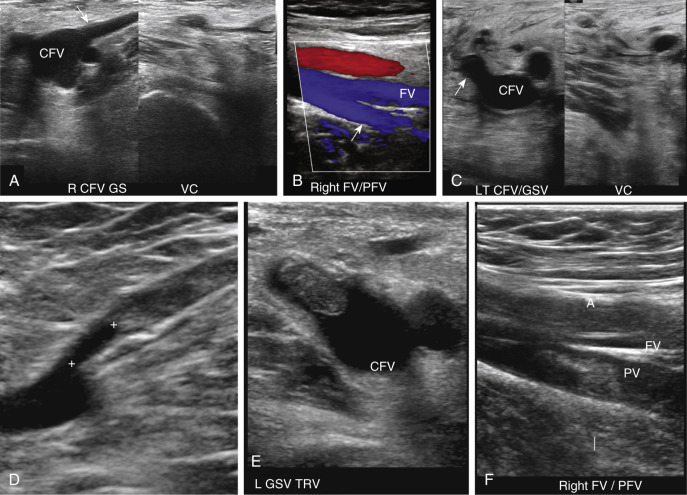
Technique
Compression ultrasound, duplex sonography, and color Doppler imaging are the diagnostic approaches of choice in cases of suspected lower extremity DVT. Sonography offers the advantages of being a noninvasive technique that does not require intravenous contrast or ionizing radiation, is available 24 hours a day, and has a relatively low cost compared with other imaging modalities. It can be performed portably at the bedside for critically ill patients, and serial follow-up exams are readily available. Properly performed, ultrasound examination of the deep veins of the thigh has proven to be a very accurate test with sensitivity and specificities of 95% and 98%, respectively.
Variations exist in the recommended technique and protocol for ultrasound examination of the lower extremities. At our institution, the patient is placed supine. The leg is externally rotated and the knee slightly bent. The head of the table can be angled 20% to 30% in reverse Trendelenburg position to promote venous pooling if necessary. Imaging is performed with a high-resolution linear transducer in the 3 to 9 MHz range. For larger patients, a curved array transducer in the 1 to 5 MHz range can be utilized to obtain greater depth penetration. With the transducer in the transverse plane, the examination begins in the groin visualizing the EIV using gray-scale imaging. Pressure is applied via the transducer to the underlying vessel to demonstrate complete compressibility of the vein. Enough pressure should be applied to fully collapse the walls of the vein. Proceeding caudally, this compression technique is used in a systematic fashion to evaluate the vessels every 1 to 2 cm. The EIV, common femoral, proximal profunda femoris, proximal great saphenous, femoral, and popliteal veins are examined using this method to the tibioperoneal trunk. The PEV is imaged with the knee flexed 20 to 30 degrees and the leg externally rotated (frog leg position). Decubitus or prone positioning with the knee flexed can also be utilized. Compression of the femoral vein in the adductor canal can be difficult especially in a large or obese patient. A “two-hand” technique is particularly helpful in these cases. The transducer is placed over the femoral vein along the medial thigh and the other hand is placed behind the adductor canal, pressing up toward the transducer until the vein collapses.
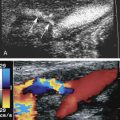
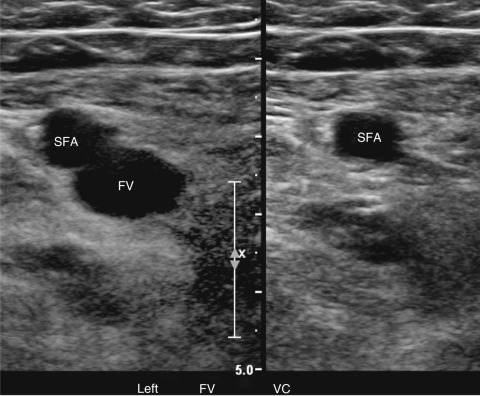
It is important to compress the vessels in the transverse plane, as compressing the vessels in the longitudinal plane may result in a false-negative result if the transducer slips off the vessel ( Fig. 19.3 ). Compression should be performed with gray-scale imaging, as color Doppler flow imaging may obscure partially occlusive thrombus and result in a false-negative examination. Cine clips can be archived to document compression. Compression maneuvers should be used judiciously if acute thrombus is present, to minimize risk of embolization to the lungs. To avoid the pitfall of mistaking low-level echoes from slow-flowing blood that mimic acute thrombus, a single compression can be performed to evaluate for collapsibility of the vessel.
Although the ultrasound examination for lower extremity DVT can be performed with gray-scale imaging alone, color Doppler flow imaging in the longitudinal plane is useful for demonstrating patent segments, detecting partially occlusive thrombus, and for identifying vessels in larger individuals that may not be seen by gray-scale imaging alone. Augmentation while searching for the veins with color Doppler flow imaging will aid in visualizing these vessels. Color Doppler images are obtained in most laboratories in both longitudinal and transverse imaging planes for all vessels.
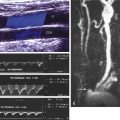
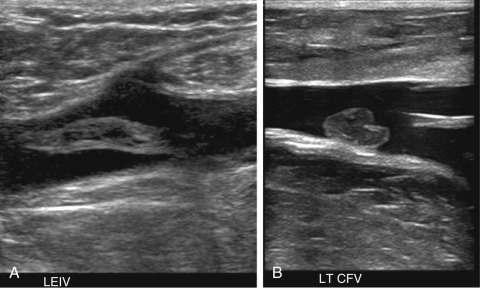
Doppler spectral waveforms should be obtained from either the EIV or CFV bilaterally even when performing a unilateral study. Normal respiratory variability (phasicity) should be seen on the Doppler waveform. Loss of phasicity indicates cephalad obstruction to flow; either thrombus in the more superior veins (e.g., CIV or IVC), venous stenosis, or extrinsic compression of the pelvic or abdominal veins by intrapelvic or intra-abdominal pathology such as a mass. All spectral waveforms should be obtained in the long-axis (longitudinal) view of the vessel.
Doppler spectral waveforms may also be obtained from the femoral and popliteal veins during augmentation maneuvers on the symptomatic side or on both sides for a bilateral examination. These maneuvers may demonstrate vein patency and improve visualization of blood flow in large patients or patients with very slow flow. Absence of an augmentation response can be utilized to confirm the presence of occlusive thrombus in venous segments not easily seen on gray-scale imaging. Augmentation should not be utilized if there is free-floating or partially occlusive thrombus noted in a vessel, as dislodgement and embolization may occur ( Fig. 19.4 ). However, a study questioned the usefulness of augmentation, noting that it does not increase the sensitivity or specificity of the exam and suggesting therefore that it can be eliminated from the lower extremity DVT protocol. In general, augmentation maneuvers do not significantly increase the time of examination and may improve determination of venous obstruction.
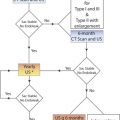
Routine evaluation of the calf veins during a lower extremity DVT examination is still a matter of debate and a clear consensus does not exist. ACR/AIUM guidelines do not require examination of the calf veins during a routine assessment. Under their guidelines, however, symptomatic areas in the calf should be evaluated with additional imaging, especially if the etiology for the patient’s symptoms is not identified on routine examination. The IAC recently changed their standards and now requires evaluation of calf veins, although the protocol and imaging requirements are not specified.
Clinical concerns may, however, necessitate the examination of the calf veins. In 2008, the American College of Chest Physicians (ACCP) recommended treatment of DVT regardless of location, including calf clot. In 2012, the ACCP modified their recommendations for testing using risk stratification strategies that included proximal or whole leg ultrasound examinations in conjunction with D-dimer testing. They also modified their treatment recommendations, stating that in patients with acute isolated distal lower extremity DVT of the leg without severe symptoms or risk factors for extension, serial imaging of the deep veins for 2 weeks is suggested over initial anticoagulation. If severe symptoms or risk factors for extension are present, anticoagulation is recommended over serial imaging. Seinturier et al. have shown that survival rates, recurrence rates, and new cancer rates in patients with VTE disease are affected by DVT location and laterality, with worse prognosis and recurrence rates for those with more proximal disease. However, a significant number of patients had poor outcomes or recurrence with distal DVT, especially for bilateral DVT.
Generally, examination of the calf veins includes the PTVs and PEVs, which are paired and accompany their corresponding named artery ( Fig. 19.5 ). The ATVs are not usually examined because of the difficulty in visualizing these veins. The PTVs and PEVs can be followed from the tibioperoneal trunk distally. If these vessels are difficult to locate superiorly, the PTVs can be identified and followed from their location posterior to the medial malleolus at the level of the ankle, and the PEVs can be located in the posterior lateral calf above the ankle. The ATVs are located anteriorly below the knee near the popliteal vessels or traced upward from the dorsalis pedis region.
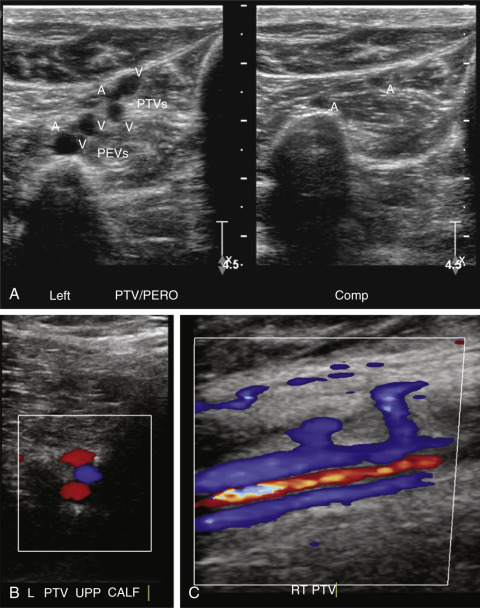
Color and power Doppler, in addition to augmentation, are used to identify and locate the calf veins, which may be small in caliber or have slow flow. Other maneuvers to improve the visualization of the veins include hanging the lower leg over the bed, placing the stretcher in reverse Trendelenburg position, and applying a tourniquet below the knee. Some authors estimate that the sensitivity of ultrasound for the detection of calf DVT is only about 60%. The sensitivity for the detection of DVT depends on the experience of the examiner, quality of the ultrasound instrument, and protocol. Therefore if the ultrasound examination is negative and there is persistent clinical concern for calf DVT, follow-up ultrasound examination in 5 to 7 days is generally advisable.
- •
The basic ultrasound examination for DVT relies on transverse compressions being applied every 1 to 2 cm along the length of the vein.
- •
Imaging of the femoral vein can be performed with the patient supine and the leg externally rotated (frog leg position).
- •
Imaging of the popliteal vein is easily done with the patient supine and the knee flexed.
- •
Gray-scale images are preferred when performing compression ultrasound.
- •
Use of color and power Doppler helps visualize blood flow when the veins are difficult to see.
- •
Augmentation maneuvers help confirm vein patency and are typically done at the popliteal vein. They are helpful when there is limited visualization of the deep veins.
- •
Repeat imaging at 2 weeks instead of anticoagulation can be performed in some patients with isolated calf vein DVT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree