The authors review ultrasound (US) imaging findings of the abdominal aorta and mesenteric arteries, with a focus on screening for abdominal aortic aneurysm (AAA) and posttreatment follow-up. US is the primary imaging modality used to screen for AAA and for surveillance of aneurysms for growth after initial diagnosis. US and contrast-enhanced US may play a greater role in patient follow-up status post-endovascular aneurysm repairs. US is also the initial recommended imaging modality for suspected chronic mesenteric ischemia.
Key points
- •
Diameter threshold of 3 cm, or 1.5 times the diameter of the more proximal abdominal aorta, is used to diagnose abdominal aortic aneurysm.
- •
Treatment using endovascular aneurysm repair or open surgical repair is indicated for abdominal aortic aneurysms 5.5 cm or greater in men, 5.0 cm or greater in women, and/or larger than 4.0 cm with a rapid increase in aneurysm size (1 cm over a 1 year period or 0.5 cm over a 6 month period).
- •
Endovascular aneurysm repair is often associated with endoleaks, with type II endoleaks being the most common.
- •
There are no widely accepted peak systolic velocity thresholds to diagnose celiac and mesenteric artery stenosis; however, a peak systolic velocity greater than 200 to 320 cm/s for the celiac artery, greater than 275 to 400 cm/s for the superior mesenteric artery, and greater than 200 cm/s for the inferior mesenteric artery are suggestive.
- •
Expiratory peak systolic velocity greater than 350 cm/s and deflection angle of greater than 50° are suggestive of median arcuate ligament syndrome.
Abdominal aortic aneurysms (AAAs) are common in the United States with significant morbidity and mortality. It is estimated that close to 200,000 cases are diagnosed per year, and rupture of an AAA is the tenth leading cause of death in men over the age of 55 years. While mesenteric arterial pathology is less common, it also is associated with significant patient morbidity. There is an increased trend toward treatment of AAA and mesenteric arterial stenoses using minimally invasive techniques, and Doppler ultrasound (US) remains the primary screening modality for aortic and mesenteric pathology as well as the initial imaging modality of choice in following patient status post-intervention. This article reviews the US imaging protocol for evaluation of the abdominal aorta and mesenteric arteries, the normal sonographic appearance of these vessels, the US imaging features of the most common pathologies affecting the abdominal aorta and mesenteric arteries, as well as the role of US in follow-up of patients who have undergone therapeutic intervention for AAA or mesenteric arterial stenosis.
Aorta
Scan Protocol
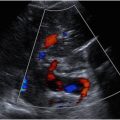
Patients are instructed to fast for 6 hours prior to imaging, as the presence of bowel gas limits evaluation of the abdominal aorta. The aorta is scanned throughout its length within the abdomen in both the longitudinal and transverse imaging planes using grayscale and color Doppler. Representative images are saved at the level of the proximal, mid (near the level of renal arteries), distal abdominal aorta, bifurcation, and the bilateral proximal common iliac arteries. The maximal diameter of the proximal, mid, and distal abdominal aorta is measured from the outer-to-outer wall in 2 orthogonal planes ( Fig. 1 ). The anteroposterior outer diameter is preferentially measured on a sagittal image with the plane of measurement-oriented perpendicular to the long axis of the lumen. The transverse outer diameter is measured on a transverse image obtained perpendicular to the long axis of the lumen. If a short-axis transverse plane cannot be obtained, the transverse diameter may be measured from a coronal image. Measurements of the bilateral common iliac arteries should be obtained in a similar fashion, as aneurysms of the iliac arteries are common in patients with AAAs. Note should be made of thrombus/plaque within the lumen of the aorta, if present. If an AAA is noted, the location relative to the renal arteries (suprarenal, juxtarenal, or infrarenal) and the aneurysm shape (fusiform vs saccular) should be described, as both location and configuration will affect patient management. If the origins of the renal arteries cannot be visualized, as a general rule, an AAA that begins more than 2 cm below the origin of the superior mesenteric artery (SMA) is most likely infrarenal.

The normal abdominal aorta is located immediately above or slightly to the left of the spinal column and descends in a straight line parallel to the inferior vena cava from the diaphragm to the pelvis where it bifurcates into the right and left common iliac arteries. The wall of the normal abdominal aorta should be smooth, symmetric, and regular. The diameter should measure less than 3 cm and taper slightly from the diaphragm to the bifurcation. The normal spectral Doppler waveform of the distal abdominal aorta is pulsatile and multiphasic without end-diastolic flow, similar to the waveforms of the common iliac arteries. The normal spectral Doppler waveform of the proximal abdominal aorta at and above the level of the renal arteries typically has a lower resistance pattern with continuous forward diastolic flow.
Abdominal aortic aneurysm
AAAs are defined as dilatation of the aorta greater than or equal to 3 cm in diameter or 1.5 times the diameter of the more proximal aorta. The incidence of AAA in men older than 60 years is estimated to be 1.7% to 3.4%. Major risk factors for AAAs include older age (>65 years), male gender, smoking, and positive family history (first-degree relative with an AAA). AAAs are 6 times more common in men than in women. Other risk factors include hypertension, atherosclerotic cardiovascular disease, and connective tissue disorders.
Although AAA may rarely present as a pulsatile abdominal mass, AAAs are usually asymptomatic until they rupture, at which time, the risk of death is upward of 80%. Accordingly, the purpose of screening is to diagnose and electively treat AAAs to reduce the incidence of AAA rupture.
Screening for abdominal aortic aneurysm
US screening has a high sensitivity (94%–100%) and specificity (98%–100%) for the detection of AAA. The United States Preventive Services Task Force (USPSTF) screening recommendations are based on current age, gender at birth, and whether the person has ever been a smoker (defined as ever having smoked 100 or more cigarettes). In 2019, the USPSTF revised its guidelines to recommend one time screening in men aged 65 to 75 years who have ever smoked. Also recommended is selective screening in men aged 65 to 75 years who have never smoked, depending on their family and medical history, other risk factors and personal values. At that time, the USPSTF concluded that the available evidence was insufficient to determine benefit of screening in women aged 65 to 75 years who have ever smoked. However, the Society of Vascular Surgery (SVS) recommends screening aortic US for both men and women aged 65 to 75 years, who have ever smoked or have a first-degree relative with an AAA. Screening is also recommended by the SVS for both men and women aged over 75 years otherwise in good health (ie, potentially interventional candidates) who have previously not been screened.
While patients with AAAs below treatment thresholds (5.0–5.5 cm) traditionally have been followed with yearly US surveillance, the SVS currently recommends follow-up US only every 3 years for AAAs 3.0 to 3.9 cm in diameter with yearly follow-up for AAAs 4.0 to 4.9 cm in size and semiannual follow-up for aneurysms 5.0 to 5.4 cm in size. Additionally, the SVS recommends a follow-up screening US in 10 years when the initial study demonstrates an abdominal aorta 2.5 to 2.9 cm in size. In follow-up studies, an increase in diameter greater than 0.5 cm is usually considered significant. The risk of AAA rupture varies with increasing aneurysm size, growth rate, and gender. Data show that women are at greater risk of rupture at smaller aneurysm size and may have a worse prognosis, even with elective intervention. Endovascular aneurysm repair (EVAR) or open surgical repair is indicated for AAAs 5.5 cm or greater in men, 5.0 cm in women, or larger than 4.0 cm with a rapid increase in aneurysm size (1 cm over a 1 year period or 0.5 cm over a 6 month period). Additionally, treatment is indicated for patients with abdominal or back pain that is attributable to the aneurysm and for all saccular aneurysms. If an AAA extends to the diaphragm, computed tomography (CT) of the chest is indicated to exclude an associated descending thoracic aortic aneurysm.
Endovascular aneurysm repair versus open repair
Multiple clinical trials have demonstrated similar long-term outcomes for EVAR compared to open repair. However, EVAR has the advantages of being less invasive, with significantly lower short-term morbidity and mortality and shorter recovery times compared to open repair. Therefore, EVAR is preferred wherever the aneurysm anatomy allows for it, which is the case in over 80% of AAAs. However, EVAR does necessitate more frequent follow-up and reintervention, especially in the case of complications, including endoleaks.
Posttreatment ultrasound surveillance
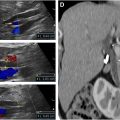
Patients with AAAs treated using EVAR need imaging follow-up at regular intervals, usually yearly for life. The most common complication following EVAR is the presence of persistent flow within the excluded aneurysm sac, termed an endoleak. Although surveillance of endografts was initially performed using CT scans, more recently, there has been increasing interest in the use of US for the detection of endoleaks due to the comparatively higher costs associated with CT scans as well as the risks associated with ionizing radiation and iodinated contrast. If the initial CT scan at 1 month following EVAR does not show an endoleak, yearly color Doppler US has been suggested as an alternative to CT scans for imaging surveillance, with subsequent CT or contrast-enhanced US (CEUS) to look for endoleak if aneurysm expansion is detected. Other complications of EVAR include migration of the graft, occlusion of a modular limb ( Fig. 2 ), and graft infection.

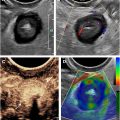
US surveillance for endoleaks must be performed with meticulous scanning technique, especially if the patient has stacked modules or chimney/snorkel graft extensions with stents placed in the upper aortic branches. Knowledge of the exact details of the endograft repair prior to US evaluation is essential to ensure that all critical components of the endograft are evaluated. The entire abdominal aorta should be evaluated first with grayscale imaging in both sagittal and transverse planes. The location/extension of the endograft should be determined and both the upper and lower landing zones well visualized if possible, assessing for gaps between the endograft and the arterial wall. Careful measurement of the maximal diameter of the aneurysm sac is critical, and the aneurysm sac should be measured at the same location on follow-up examinations. Although not all endoleaks result in continued growth of the aneurysm, increased aneurysm size is one of the most important findings in patients with endoleaks, usually indicating that repair is necessary, no matter the type of endoleak. Anechoic areas within the excluded sac should be assessed with color Doppler and microvascular flow imaging (MVFI), as endoleaks are often anechoic (without thrombus). Subsequently, careful evaluation of the entire aneurysm and endograft in both sagittal and transverse planes should be made with color Doppler and MVFI to document graft and outflow limb patency. Areas of color flow in the excluded aneurysm sac are indicative of endoleak, including at gaps between endograft and aortic/iliac artery wall, retrograde flow in a feeding vessel, or flow extending through the wall of the graft into the aneurysm sac. Color Doppler and MVFI cine clip imaging in both sagittal and transverse planes are very useful.
Types of endoleaks are described in Table 1 . Type I endoleaks reflect an incomplete seal between the endograft and the vessel wall, most likely due to endograft migration or continued growth or deformation of the aneurysm. A gap between the wall of the aorta or iliac artery and the endograft at the landing zone may be observed on grayscale with flow coursing between the graft and vessel wall on color/power Doppler. A type IA endoleak occurs at the level of the cephalad landing zone and a type IB endoleak occurs at the distal landing zone in the iliac arteries. Type I endoleaks are usually large. Flow may slowly swirl within the endoleak on grayscale and demonstrate a “yin-yang” pattern on color Doppler, similar to flow in a pseudoaneurysm.
| Type | Cause | Management Considerations |
|---|---|---|
| I | Inadequate seal of the endograft to aortic wall | Subtypes IA and IB due to inadequate seal proximally (IA) and distally (IB). Usually treated |
| II | Retrograde flow in aortic branch arteries, usually inferior mesenteric or lumbar arteries | Common finding : Usually treated only if symptomatic, or if the aneurysm increases in size > 5–10 mm |
| III | Mechanical graft failure with blood flow between graft components, fabric tear, or separation of modular components | Typically require intervention to repair |
| IV | Graft material porosity | Less commonly seen with current devices. Usually seal spontaneously, and are not treated. |
| V | Aneurysm enlargement in the absence of an endoleak visible by imaging | Treatment is recommended |
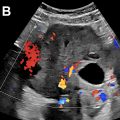
Type II endoleaks are the most common type, and these are found in approximately 25% of patients undergoing EVAR. On grayscale imaging, type II endoleaks often appear as small, round anechoic areas in the excluded aneurysm sac, and will demonstrate flow on color or power Doppler ( Fig. 3 ). US can often demonstrate retrograde flow into the endoleak from the patent inferior mesenteric artery (IMA) anteriorly or a lumbar artery posteriorly, confirming a type II endoleak. The spectral Doppler waveform obtained from the feeding branch typically has a “to-and-fro” waveform pattern, similar in appearance to the flow in the neck of a pseudoaneurysm. If persistent forward diastolic flow is observed in the feeding vessel, the possibility of a complex endoleak with either a second feeding vessel or an associated type I or type III endoleak should be considered, and follow-up CT may be helpful.