Ultrasound (US) plays a primary role in the assessment and diagnosis of renal and ureteral pathologies and their management. It is considered the first-line imaging modality for evaluation of urinary obstruction, nephrolithiasis, and urinary retention among other indications. US is also essential for evaluation of renal vasculature and assessment of renal transplantation. Contrast-enhanced US is an advanced application of US gaining its acceptance in evaluation of the renal masses.
Key points
- •
Conventional and advanced ultrasound (US) plays a primary role in the assessment and diagnosis of the kidneys and bladder pathologies and their management.
- •
US is the first-line imaging modality for the evaluation of urinary obstruction, nephrolithiasis, renal vascular pathologies, and genitourinary infection with advanced techniques such as contrast-enhanced US gaining popularity in the diagnosis of renal malignancies; and shear wave elastography emerging as a technique for evaluation of the degree of chronic renal fibrosis.
- •
Conventional US is the modality of choice in evaluation of urinary bladder masses, causes of bladder outlet obstruction, and post treatment follow-up of urinary retention.
Introduction
Conventional and advanced ultrasound (US) techniques are essential in evaluation of a number of renal and ureteral pathologies ( Table 1 ). Kidney diseases can result from a wide range of genetic, hemodynamic, toxic, infectious, and autoimmune factors. Although the diagnosis of kidney disease usually involves analysis of clinical presentation, serum blood, and urine, these parameters are often insufficient to make a definitive diagnosis. US imaging as a first look can narrow down the diagnosis and guide choices regarding additional imaging and overall patient management.
| Grade of Hydronephrosis | Characteristic Appearance |
|---|---|
| Grade 0 | No hydronephrosis |
| Grade 1 | Dilatation of renal pelvis only |
| Grade 2 | Dilatation of renal pelvis and dilatation of a few calyces |
| Grade 3 | Dilatation of renal pelvis and dilatation of all calyces |
| Grade 4 | Dilatation of renal pelvis, dilatation of all calyces, and thinning of the renal parenchyma. |
US is non-invasive, portable, and safe; it does not utilize ionizing radiation or intravenous iodinated contrast material, and can detect structural, functional, and hemodynamic changes within the kidney. New methods, such as contrast-enhanced US (CEUS) and elastography can allow more accurate assessment of renal masses and chronic renal disease. , Microbubbles can also help assess renal vascular patency and cortical perfusion in native and transplanted kidneys. When utilized, these methods greatly advance the diagnosis of renal and bladder diseases and safe monitoring of patients with serial follow-up examinations.
This article reviews the role of US imaging in the evaluation of suspected renal and ureteral disorders with emphasis on key sonographic features, utility of Doppler, and advanced techniques such as microflow imaging and CEUS.
Anatomy
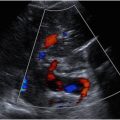
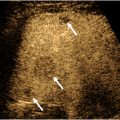
Kidneys have a relatively complex anatomy. Depending on the patient size, gender, and age, the kidneys range in size from 9 to 13 cm. Atrophic kidneys usually measure less than 8 cm in length and demonstrates heterogeneous and echogenic renal parenchyma (see Fig. 3 ). On US, the bean-shaped kidneys consist of fibrofatty echogenic renal sinus and relatively hypoechoic renal cortex. The renal cortex is slightly more hypoechoic than the liver and substantially more hypoechoic than the spleen. Heart-shaped pyramids are either isoechoic or slightly hypoechoic relative to the renal cortex ( Figs. 1 and 2 ). In patients with medical renal disease, the echogenic renal cortex is easy to characterize relative to the adjacent liver parenchyma ( Fig. 3 A, B ). Echogenic kidneys are considered a “nonspecific finding” and can occur with other renal conditions such as chronic renal failure, drug-induced toxicity, metabolic disease, and infection. The intrarenal collecting and vascular systems travel through the echogenic renal sinus and may be seen as tubular anechoic structures converging at the renal hilum if dilated. Although the surface of the kidneys is generally smooth, a common variant, called the junctional parenchymal defect, can produce a wedge-shaped indentation on the surface of the kidney near the junction of the upper and middle thirds of the renal cortex. This incomplete embryologic fusion of the upper and lower poles can be confused for scarring, traumatic injury, or a mass. Triangular shape and location of the defect are the keys to differentiate it from pathologic processes. A central wall of cortical tissue ( Column of Bertin ) can protrude into the renal sinus simulating a mass ( Fig. 4 ). , Location in the mid third of the kidney, identical echogenicity as the renal cortex, uninterrupted continuation of the vessels into the tissue, and occasionally presence of a small hypoechoic pyramid are features that aid in differentiation of the column from pathologies (see Fig. 4 A–C). Another common variant is duplication of the intrarenal collecting system , characterized by central band of cortex between the upper and lower pole ( Fig. 5 A, B ).





Congenital anomalies
Kidney agenesis is a rare congenital anomaly of the kidneys, where one of the kidneys is not developed resulting in empty renal fossa, contralateral kidney hypertrophy and linear shape of the ipsilateral adrenal gland rather than V-shaped configuration. It is associated with anomalies of the Mullerian duct. In women it can be seen with morphologic anomalies of the uterus and in man it may present with seminal vesicle anomaly or anomaly of the vas deferens. Ectopic kidney can be positioned anywhere in the abdomen and even thorax and usually has a blood supply from the adjacent vessels ( Fig. 6 A, B ). In contrast, a ptotic kidney is located lower than expected but still maintains the vascular supply from the abdominal aorta with the main renal artery originating at the same level as the normally positioned kidney. Crossed fused ectopia can also occur and may present as a very large kidney in a renal fossa with empty contralateral renal fossa. The ectopic kidney is fused with its upper pole to the lower pole of normally positioned kidney ( Fig. 7 A–D ). The ureters maintain 2 normal urinary bladder insertion sites, and vascular supply is not altered. Horseshoe kidney is one of the most common fusion anomalies characterized by a variable thickness band of renal tissue (isthmus) extending from both lower poles to connect anterior to the aorta below the level of the inferior mesenteric artery ( Fig. 8 ).



Principles of examination and technique
There are several key elements to a successful renal examination. Although usually patients do not need to fast for general kidney assessment, adequate patient preparation can be essential when evaluating renal vasculature, for example, in the setting of suspected renal artery stenosis (RAS) or vascular thrombosis. Fasting can also decrease amount of bowel gas, improving visualization of the deeply positioned kidneys.
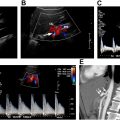
Curvilinear (2 – 5 MHz) transducers are used to maximize US beam penetration. Grayscale, color, spectral, and power Doppler are utilized for general assessment of the kidneys. Various parameters are optimized for better visualization of the examined structures, including but not limited to acoustic window, depth, brightness, focal zone, velocity scale, wall filter, and angle of insonation. When pathology is suspected, other more advanced US techniques and applications such as B-flow, microflow imaging, and CEUS can be helpful for more definitive diagnosis, especially for the assessment of vascular patency, mass vascularity, or kidney parenchymal perfusion in native kidney and renal transplants. Elastography can also improve confidence in the assessment of chronic and acute renal failure.
The US examination is performed with the patient in the supine position. The kidneys are examined in longitudinal and transverse scan planes with the transducer placed on the flanks using liver and spleen as acoustic windows. Supine and the lateral decubitus positions can be included when visualization of the kidney is obscured by bowel gas, with the transducer moved dorsally.
Nonvascular renal disorders
Obstruction
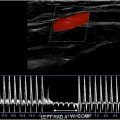
One of the most common indications for renal US is renal insufficiency or suspected obstructive uropathy. In most cases, bilateral obstruction is required for renal insufficiency to develop. Untreated obstruction may result in irreversible renal functional impairment, thus necessitating prompt diagnosis and management. Obstruction can be due to variable etiologies, including but not limited to obstructive stone, mass, external lymphadenopathy, abscess, pelvic mass/neoplasm, retroperitoneal fibrosis, among other etiologies. Hydronephrosis refers to dilatation of the intrarenal collecting system, including calyceal and pelvic dilatation. Hydroureteronephrosis describes dilation of the intrarenal and extrarenal collecting systems, including dilation of the ureters (≥5 mm in diameter) ( Fig. 9 A, B ). It is important to note that 3 mm should be considered the upper limit of normal size for unobstructed ureters on unenhanced helical computed tomography (CT). The overall reported diagnostic accuracy of US in detecting hydronephrosis is 85.2%, with a specificity of 84.4% and a sensitivity of 89.9%. In addition to its ability to detect hydronephrosis, US also helps to differentiate acute obstructive hydronephrosis from non-obstructive hydronephrosis (for example physiologic hydronephrosis from gravid uterus, or persistently dilated collecting systems due to previous episodes of obstruction, or active physiologic diuresis, or overdistension of the urinary bladder and vesicoureteral reflux). All of these processes will show dilated collecting systems but without obstructive cause. The sensitivity of US in detecting urinary tract obstruction is 93%. On US, hydronephrosis can be differentiated from peripelvic cysts by demonstrating convergence of the dilated calices to the level of the dilated renal pelvis, resembling a glove appearance with all the intrarenal segments communicating with the renal pelvis. Spectral Doppler can increase confidence in the diagnosis of obstructive hydronephrosis by demonstrating relatively elevated resistive index (RI), although this concept is still controversial as many processes may result in elevated RI. There are 5 grades of obstruction recognized ranging from slight expansion of the intrarenal collecting system to end-stage hydronephrosis with cortical thinning ( Table 1 ). In the setting of long-standing obstructive uropathy, the renal cortex becomes thin allowing differentiating chronic from acute obstruction ( Fig. 10 ). Color Doppler can help distinguish prominent renal vessels from the collecting system ( Fig. 11 A–D ). Due to bowel gas distension obscuring evaluation of the mid and lower pelvic organs, only the proximal and the most distal segments of the ureter are commonly visualized on US. Identification of an echogenic focus within the lumen with posterior shadowing and upstream ureteral dilatation can help to make accurate diagnosis (see Fig. 11 ). The mid segment of the ureter is rarely seen on US unless it is severely dilated and tortuous. Doppler US can improve renal stone visualization and confidence by demonstrating a twinkling artifact. , Analysis of ureteral jets using Doppler can assist in the evaluation of potentially obstructed kidney and assess the degree of obstruction. Thus, in the presence of obstruction, ureteral jets usually are absent or in the case of partial obstruction, the jets can be markedly diminished. In the setting of nonobstructive hydronephrosis, the ureteral jets are maintained. US is the most optimal imaging modality for serial follow-up of the patients with obstructive stones to monitor for relief of obstruction, which would be suggested by reappearance of the ureteral jets and resolution of hydronephrosis. Urolithiasis is the most common cause of hydronephrosis in the adult patient and has a prevalence of 10% – 15%. In a study of 210 patients with hydronephrosis, B-mode US imaging determined the cause of hydronephrosis in 65.2% of cases with urolithiasis being the cause of obstruction in 60% of the patients. The detection rate of urinary stones was 50%, 61%, and 71.4% for grades 1, 2, and 3 hydronephrosis, respectively. When an obstructive etiology is not identified on US, further imaging with CT urography is required. Presence of hematuria and obstruction without renal stones should raise concern for an underlying neoplasm and warrant further imaging assessment using CT or magnetic resonance (MR) urography, which allow evaluation of the entire upper and lower collecting systems ( Fig. 12 A–C ). If the dilated calyces show internal debris, infection such as pyonephrosis is suspected, and correlation with urinalysis and clinical presentation should be made ( Fig. 13 A, B ). Pyonephrosis can cause rapid and permanent deterioration of renal function and should be decompressed as soon as it is detected.





Cystic Diseases
Renal cysts are the most frequently encountered renal lesions. They are seen in 50% of people over age of 50 y. They may increase in size and become complex due to development of septations, precipitation of milk of calcium in the wall, and internal hemorrhage. The main US characteristics of a simple renal cyst include anechoic lumen, well-defined back wall, acoustic posterior enhancement, lack of internal flow, and imperceptible avascular wall (too thin to measure) ( Fig. 14 ). Harmonic imaging and real-time compounding can aid in minimizing internal artifacts within the cysts, which can be encountered by imaging the cysts from different approaches.

By location, renal cysts can be divided into the cortical and parapelvic (originating in the renal sinus, commonly multiple) (see Fig. 14 ; Fig. 15 A, B ). Doppler US helps to differentiate the renal cysts from aneurysms, pseudoaneurysms (PSAs), arteriovenous fistulas, and vascular cystic neoplasms by demonstrating characteristic flow patterns and specific waveforms within these structures.

Complex cysts are those with variable-thickness septations or filled with echogenic material and/or solid components. Number and thickness of the septations and presence of nodular components determine complexity of the cysts. Hemorrhagic cysts may show either a retracted clot or low amplitude echogenic material, and on the serial follow-up examinations will show evolution of the hemorrhage often resulting in decreased size of the cyst. When large in size, hemorrhagic cysts rarely can rupture and become superinfected ( Fig. 16 A–C ). Proteinaceous cysts may also show echogenic material within but do not morphologically change with time. Calcifications occur in 1% to 3% of cysts as a sequela of prior infection, hemorrhage, or ischemia. Thick globular calcification should raise suspicion for malignancy. CEUS has emerged as an alternative imaging modality compared to CT angiography and MR angiography (MRA) for evaluation of suspected complex renal cystic lesions suspicious for neoplasms. Presence of enhancing components within the cystic renal lesions or septal enhancement should increase confidence in the diagnosis of a neoplasm.

Multiple cysts are usually seen in conditions such as autosomal dominant polycystic kidney disease (ADPKD), acquired cystic renal disease, von Hippel-Lindau disease (VHL), and tuberous sclerosis (TS).
In ADPKD, the various size and complexity cysts are seen in the bilateral enlarged kidneys occupying the renal cortex and medulla, and in more severe cases they may cause compression of the collecting systems. Urine stasis resulting in solid appearance of the cysts due to formation of stones or hemorrhage are additional hallmarks of this condition ( Fig. 17 A–C ).