Positron Emission Tomography/Computed Tomography (PET/CT) plays a critical role in managing gastrointestinal (GI) cancers within radiation oncology. It enhances tumor detection, staging, and lymph node involvement assessment, leading to better-targeted radiation treatment. PET/CT also aids in delineating tumor volumes to minimize geographic misses, enabling precise dose escalation to metabolically active regions. Despite its benefits, PET/CT has limitations such as false positives and dependency on complementary imaging. Emerging technologies offer real-time adjustments and personalized treatments, advancing precision medicine in GI radiation oncology. Further research is needed to refine PET/CT integration for improved treatment outcomes and cost-effectiveness.
Key points
- •
Positron Emission Tomography/Computed Tomography (PET/CT) enhances the detection of lymph node involvement and metastases, which is crucial for accurate staging and guiding treatment decisions.
- •
PET/CT improves tumor and lymph node delineation in GI cancers, reducing geographic misses and enabling precise targeting and dose escalation in metabolically active areas.
- •
PET/CT provides prognostic information and allows for early treatment response monitoring, can be used for adjustments in radiation therapy plans.
- •
PET/CT has limitations, including potential false positives/negatives. Pathologic correlation and complementary imaging, like endoscopic ultrasound and MR imaging, are essential for validation and accuracy.
- •
PET/MR imaging and PET/linear accelerator are advancing GI radiation oncology, offering improved tumor delineation, real-time treatment adjustments, and personalized therapy, representing the future of precision medicine.
| BRPC | borderline resectable pancreas cancer |
| cCR | clinical complete response |
| CRT | chemoradiation |
| CT | computed tomography |
| EUS | endoscopic ultrasound |
| GI | Gastrointestinal |
| LARC | locally advanced rectal cancers |
| LINAC | linear accelerator |
| OS | overall survival |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| RPC | resectable pancreas cancer |
| RT | radiation therapy |
| SBRT | stereotactic body radiation therapy |
| SUV | standard uptake value |
Introduction
Gastrointestinal (GI) cancers are increasing worldwide and are often associated with high mortality rates. The oncological outcomes have improved in the last few decades with improvement in diagnostic and therapeutic strategies. Positron emission tomography/computed tomography (PET/CT) has become an essential tool in the management of GI malignancies. By combining the metabolic imaging capabilities of PET with the anatomic detail of computed tomography (CT), PET/CT offers enhanced precision in tumor detection, staging, treatment planning, and response assessment. The role of PET/CT in radiation oncology has expanded; and it impacts treatment decisions, radiation planning, prognosis, and monitoring of treatment response. In GI radiation oncology, PET/CT plays a pivotal role as a complementary imaging modality, particularly when combined with high-quality contrast-enhanced CT or MR imaging. This integration, along with other imaging techniques such as MR imaging and endoscopic ultrasound (EUS), enhances the accuracy of tumor and nodal delineation, essential for precise radiation treatment planning. This review explores the current applications, benefits, and limitations of PET/CT in the radiation oncology management of GI cancers, including esophageal, gastric, pancreatic, rectal, and anal cancers.
Role of PET/computed tomography in gastrointestinal radiation oncology
PET/computed tomography significantly enhances the detection and staging of GI malignancies. It is particularly effective in the early detection of lymph node involvement, skip lesions, peritoneal metastases, and oligometastases, aiding in distinction between benign and malignant processes and local versus distant disease states. This is crucial for accurately staging the disease and guiding treatment decisions, such as surgical planning, neoadjuvant therapies, postoperative management, and metastasis-directed therapies. Additionally, PET/CT can be helpful in patients with contrast allergy or with renal disease who cannot tolerate contrast.
In radiation therapy (RT) planning, PET/CT improves target volume delineation, especially for primary and nodal diseases, facilitating more accurate contouring, and allowing for more precise radiation boost treatment. It decreases the inter-observer variation in target volume delineation. It is also valuable in stereotactic body radiation therapy (SBRT) planning and determining whether dose escalation or deescalation is appropriate. PET/CT is also used for focal increase in dose to the areas with active disease with simultaneous integrated boost or dose-painting, while minimizing exposure to surrounding healthy tissues. PET/CT may allow for adaptive radiotherapy in future, where treatment plans can be modified based on the tumor’s metabolic response observed during interim scans. PET/CT may also be helpful in the consolidative setting when attempting to identify regions at risk for residual microscopic disease.
In addition to planning, PET/CT is instrumental in monitoring treatment response and detecting recurrence and metastases. It can identify changes in tumor metabolism earlier than traditional size-based assessments, which is valuable in adjusting treatment plans and predicting patient outcomes. Prognostically, parameters such as standard uptake value (SUV), metabolic tumor volume, and total lesion glycolysis have been linked to overall survival (OS) and disease-free survival in GI cancers, though these correlations vary across different studies.
Despite its advantages, PET/CT has limitations, including the potential for false positives and negatives, the need for pathologic correlation to validate imaging findings, and concerns about cost-effectiveness. It is less reliable for detecting small, superficial, or highly mucinous tumors, which may exhibit low fluorodeoxyglucose (FDG) uptake and therefore be missed on PET/CT. In these cases, EUS remains the preferred modality for staging as it provides superior visualization of the tumor and its relationship to adjacent structures, as well as better detection of lymph node involvement. Continued research is essential to further understand the long-term impact of PET/CT on treatment outcomes, optimize its use in clinical practice, and evaluate its economic viability in managing GI malignancies.
PET/computed tomography in esophageal and gastroesophageal junction cancers
Tumor Delineation and Geographic Misses
Accurate tumor delineation is a cornerstone of effective radiation therapy. In esophageal and gastroesophageal junction (GEJ) cancers, where the tumor often extends along the length of the esophagus and involves adjacent structures, precise delineation is crucial to ensure complete tumor targeting while sparing healthy tissues. PET/CT has demonstrated significant advantages over CT alone in this regard, particularly in defining the gross tumor volume (GTV) and skip lesions. A study by Jimenez and colleagues emphasized the importance of incorporating PET/CT into radiotherapy planning for esophageal cancer, highlighting its ability to significantly increase the lymph node coverage compared with CT. The study also found that PET/CT improved the accuracy of delineating the cranial and caudal borders of the tumor, thereby reducing the risk of geographic misses and improving the dose coverage of the tumor in the radiation field. This enhanced delineation is particularly important in the upper and lower esophagus, where the proximity of critical structures like the lungs and heart makes precise targeting essential.
Lymph Node Involvement and Distant Metastasis
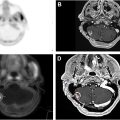
PET/computed tomography is superior to CT in identifying metastatic nodes and distant lesions and is recommended by guidelines to exclude metastatic lesions. This capability is particularly valuable in cases where the disease has spread beyond the primary tumor site. Chatterson and colleagues evaluated 129 patients with esophageal cancer without definite distant metastases in a prospective multicenter trial and showed that PET identified metastatic sites in 41% of cases and altered management in 38% of cases. This upstaging can have a profound impact on treatment strategies, often shifting the therapeutic approach from curative to palliative or modifying the sequencing of therapies. Fig. 1 shows PET/CT image of a patient with lower thoracic esophagus with mediastinal lymph nodes (LN).

Impact on Radiation Volumes
By providing more accurate delineation of the tumor and its extension into adjacent tissues, PET/CT allows for more precise radiation targeting, potentially reducing the radiation dose to surrounding healthy organs such as the lungs and heart. Jimenez and colleagues demonstrated that PET/CT-based planning could lead to dose escalation within the tumor while minimizing the dose to adjacent organs. Similarly, the radiation target volumes in esophageal cancer changes significantly based on nodal involvement in supraclavicular, mediastinal or celiac LN. For example, a primary tumor in mid-lower esophagus with mediastinal node involvement on PET/CT will necessitate the coverage of mediastinal and supraclavicular nodal regions as per the esophageal cancer contouring guidelines. Fig. 1 shows images of a patient with lower esophageal cancer who underwent PET, which showed avid mediastinal LN and led to change in treatment volumes to include the mediastinal and bilateral supraclavicular regions.
PET/Computed Tomography in Treatment Planning and Decision-Making
PET/computed tomography plays a crucial role in guiding treatment decisions for esophageal and GEJ cancers, particularly in the context of surgical planning and neoadjuvant therapy—chemotherapy or chemoradiation (CRT). By accurately staging the disease, PET/CT helps to tailor treatment strategies to individual patients. In CALGB 80803, early PET assessment individualized treatment of esophageal and GEJ adenocarcinoma patients randomized to induction oxaliplatin, leucovorin, and fluorouracil (FOLFOX) vs carboplatin-paclitaxel. PET non-responders (<35% SUV decrease) switched to the alternative chemotherapy during CRT, while PET responders (≥35% SUV decrease) continued the same regimen. Median OS was 48.8 months for responders vs 27.4 months for non-responders (HR 1.34, 95% CI 0.94–1.92), highlighting the prognostic importance of PET. In non-responders patients who switched to alternate chemotherapy per PET guidance, pCR was 18% to 20% which is similar to 14% pCR in carboplatin-paclitaxel responders, however, pCR in FOLFOX responders was high to 40.3%.
PET-response based tailoring of treatment originally was explored in the MUNICON study from Germany in locally advanced esophageal and GEJ cancers. In this phase II study, patients (n=110) received 2 cycles of induction chemotherapy with platinum and fluorouracil. PET non-responders (<35% SUV decrease) discontinued chemotherapy and proceeded to surgery, while PET responders (≥35% SUV decrease) continued chemotherapy for 12 weeks followed by surgery. Median event-free survival was 14.1 months in non-responders vs 29.7 months in responders ( P = .002). Median OS in non-responders was 25.8 months, while median survival was not reached in responders (HR 2.13, 95% CI 1.14–3.99, P = .015). Major histologic remissions (<10% residual tumor) were noted in 58% of responders vs 0% in non-responders. This study showed usefulness of early metabolic evaluation in tailoring treatment and led to MUNICON II study to improve the clinical outcome of metabolic non-responders using a neoadjuvant radiochemotherapy. In this phase II study (n = 56), the metabolic non-responders (n = 23) after 2 weeks of induction chemotherapy received neoadjuvant CRT followed by surgery while metabolic responders (n = 33) continued 12 weeks of chemotherapy followed by surgery. R0 resection rate was 82% vs 70% ( P =.51), however, 26% of non-responders had major histologic remissions. One-year PFS was 74% in metabolic responders vs 57% in metabolic non-responders ( P =.035). Thus, PET response based tailoring of treatment may improve outcomes in poor risk patients and provides prognostic information also.
One of the strengths of PET/CT is its ability to identify patients who achieve a complete response to neoadjuvant CRT, especially in squamous cell carcinoma in upper or middle esophagus. In a prospective, multicenter, diagnostic cohort study (pre-SANO study) by Noordman and colleagues, 219 patients of cancer esophagus treated with neoadjuvant CRT underwent response evaluation with EUS, biopsy, FNA and PET/CT prior to esophagectomy. PET/CT missed 6 of 41 (15%) of tumor regression grade 3/4 patients and was able to detect interval distant metastases in 9% of cases. This led to development of SANO trial, which is a phase III multicenter, stepped-wedge cluster randomized controlled trial in which patients with locally advanced esophageal cancer with clinical complete response (based on EUS, biopsy, FNA and PET/CT) after neoadjuvant CRT were randomized to immediate surgery verses continued surveillance. Thus, this study opens the role of PET/CT in non-operative management of esophageal cancer, especially in the squamous cell cancer settings. , In such cases, PET/CT can potentially avoid unnecessary surgery by indicating that the tumor has been effectively treated by the neoadjuvant treatment. This is particularly relevant in the context of the “watch-and-wait” approach, where surgery may be deferred in patients with a complete metabolic response on PET/CT. Conversely, in patients who do not respond adequately to neoadjuvant CRT, PET/CT can guide further treatment decisions, such as the need for additional therapy or a change in the surgical approach.
PET/computed tomography in gastric cancers
Role in Disease Staging/Evaluation
PET/CT demonstrates a lower accuracy rate in gastric cancer due to reduced FDG uptake, particularly in diffuse and mucinous tumor types. While it shows lower sensitivity than CT for detecting local lymph node involvement (56% vs 78%), it offers greater specificity (92% vs 62%). In preoperative staging, PET/CT has a notably higher accuracy rate (68%) compared with CT alone (53%). However, PET/CT cannot replace staging laparoscopy, as it fails to reliably detect peritoneal disease. However, PET/CT can be complemented by EUS to provide local tumor stage and contrast-enhanced CT to ensure a more comprehensive assessment of the disease. PET/CT has shown promise in the management of advanced-stage gastric cancer, and is valuable for detecting distant metastases, which are critical for accurate staging and determining the appropriate treatment strategy.
Tumor and Target Volume Delineation
Radiation therapy in gastric cancer can be considered in preoperative settings or in the post-op adjuvant settings as per the pathologic risk factors. PET/CT can help in RT planning by improving the definition of GTV in gastric cancer, where the tumor may involve multiple regions of the stomach and adjacent lymph nodes. PET/CT helps identify metabolically active tumor regions and LN that might not be visible on CT alone, thereby enhancing the accuracy of target delineation. Inclusion of nodes and extent of nodal coverage in the radiation field is a key consideration in the treatment of gastric cancer. PET/CT can help optimize the radiation dose distribution, balancing the need to eradicate the tumor while minimizing exposure to surrounding healthy tissues.
Restaging After Neoadjuvant Therapy
PET/CT is also valuable in restaging gastric cancer after neoadjuvant therapy, particularly in cases where the disease remains unresectable. By assessing the metabolic response to therapy, PET/CT can provide critical information on whether the tumor has become operable or if further systemic or radiation treatment is required. This helps in tailoring the treatment plan based on the tumor’s response.
PET/computed tomography in pancreas cancers
Role in Disease Staging/Evaluation
Pancreatic protocol triphasic CT is preferred for its ability to precisely visualize the primary tumor’s relationship to mesenteric vasculature and detect sub-centimeter metastatic deposits. However, its sensitivity for small hepatic and peritoneal metastases is limited. The pancreas protocol MR imaging with contrast is a valuable adjunct to CT in pancreatic cancer staging, especially for characterizing indeterminate liver lesions or identifying tumors not visible on CT. While PET/CT is not a substitute for high-quality contrast-enhanced CT, it can be considered an adjunct in high-risk patients with equivocal imaging findings, elevated CA 19-9, large primary tumors, significant regional lymphadenopathy, excessive weight loss, and extreme pain. A retrospective study found that PET/CT following a standard CT protocol increased sensitivity for detecting metastatic disease compared with standard CT or PET/CT alone. The sensitivity for detecting metastases was 61% for PET/CT alone, 57% for standard CT alone, and 87% for the combination. PET/CT findings altered the clinical management of 11% of patients with invasive pancreatic cancer.
Neoadjuvant treatment in pancreas cancer is evolving with improved outcomes in resectable pancreas cancer (RPC) and borderline resectable pancreas cancer (BRPC) in the PREOPANC trial. In this multicenter phase III trial, 246 patients with RPC and BRPC were randomized to neoadjuvant CRT (3 cycles of high-dose gemcitabine with 36 Gy in 15 fractions during the second cycle) or upfront surgery. The neoadjuvant CRT group showed improved OS (HR, 0.73; 95% CI, 0.56–0.96; P =.025), with a 5-year OS rate of 20.5% versus 6.5% for upfront surgery. The results of SBRT are evolving in the neoadjuvant settings with inferior results in the phase II Alliance A021501 trial, however encouraging results in SMART trial with adaptive RT. In these scenarios, PET/CT can be used before and after neoadjuvant therapy to assess response, restage the disease, and tailor the treatment.
PET/Computed Tomography in Tumor Delineation and Treatment Planning
Given the proximity of the pancreas to critical structures, precise tumor delineation is crucial to maximize the therapeutic dose to the tumor while minimizing exposure to surrounding healthy tissues. PET/CT enhances tumor delineation by identifying metabolically active tumor regions that may be overlooked by CT alone. Topkan and colleagues reported that the integration of PET/CT in radiation therapy planning led to significant increases in both GTV and planning target volume (PTV) in patients with locally advanced pancreatic cancer. This is particularly important in cases where the tumor extends beyond the boundaries visible on CT, as PET/CT can detect metabolically active regions that might otherwise be excluded from the radiation field.
Prognostic Value of PET/Computed Tomography
PET/CT is increasingly recognized for its prognostic value in pancreatic cancer. Studies have indicated that PET/CT parameters, such as the SUVmax, may correlate with overall survival and disease progression. , A higher SUVmax, reflecting greater metabolic activity within the tumor, has been associated with poorer prognosis, suggesting that PET/CT could be used to stratify patients according to their risk and guide treatment decisions accordingly. , In a retrospective study of 102 patients with RPC or BRPC, Suto and colleagues demonstrated that ≥60% reduction in SUVmax on FDG-PET after neoadjuvant CRT correlated with significantly improved RFS compared with non-responders (HR 7.30; 95% CI 2.10–25.40, P =.002) and pathologic response, suggesting response assessment with PET CT after NACRT can be of prognostic and predictive value.
Monitoring Treatment Response
By assessing changes in FDG uptake before and after therapy, PET/CT can provide early indications of how well the tumor is responding to treatment. A systematic review by Evangelista and colleagues indicated that PET/CT can be useful for predicting and assessing response to CRT in patients with RPC or BRPC. This can be particularly useful in adapting radiation therapy plans, such as by escalating the dose to regions of the tumor that remain metabolically active or by adjusting the treatment approach if the tumor is not responding as expected. This ability to monitor and adjust treatment in real-time underscores the potential of PET/CT to improve outcomes in patients with pancreatic cancer.
PET/computed tomography in rectal cancers
Role in Disease Staging/Evaluation
RT is routinely used in the neoadjuvant setting in locally advanced rectal cancers (LARC), and the trend is toward total neoadjuvant therapy (TNT) and non-operative management (NOM). , Thus, accurate primary tumor and LN contouring is essential in radiation therapy to ensure that the entire tumor is effectively targeted while minimizing radiation exposure to surrounding healthy tissues. In rectal cancer, precise delineation of the GTV is particularly challenging due to the tumor’s proximity to critical structures such as the bladder, small intestine, and pelvic bones. Geographic misses—where parts of the tumor are inadequately covered by the radiation field—can lead to local recurrence and reduced treatment efficacy. PET/CT provides metabolic information that complements the anatomic details obtained from CT/MR imaging. Buijsen and colleagues reported that PET/CT led to increase in inter-observer agreement in the GTV definition in rectal cancer, avoided geographic misses, and allowed tailoring the caudal border of the treatment volume. A prospective study by Agarwal and colleagues of 44 patients with rectal cancer with lateral pelvic LNs on MR imaging and negative systemic metastases on conventional imaging showed that PET detected additional extra-pelvic metastases in 11.4% and altered treatment plans in 15.9% of cases. PET can also be used in selected cases to assess potential metastatic disease, particularly in settings of elevated tumor markers.
Radiation Boost or Dose Escalation in Rectal Cancer
PET/computed tomography along with MR imaging can be particularly useful in identifying the high-risk areas, ensuring that the radiation boost is accurately targeted. This approach can be especially beneficial in patients with locally advanced rectal cancer, where achieving complete tumor control is crucial for improving long-term outcomes. By accurately delineating these regions, PET/CT allows for targeted dose escalation, potentially improving treatment outcomes while minimizing the risk of toxicity to adjacent healthy tissues.
Implications for the Watch-and-Wait Protocol
In the context of the watch-and-wait protocol, where surgery is deferred in favor of close monitoring, the accuracy of GTV delineation becomes even more critical. PET/CT can contribute valuable information about the tumor’s borders, ensuring that the radiation dose is sufficiently targeted to achieve tumor control without unnecessary exposure to surrounding tissues. Studies indicate approximately 20% 2-year local recurrence rate for LARC patients with clinical complete response (cCR) following a watch-and-wait approach. While MR imaging and endoscopy with biopsy are typically used to assess cCR, improved evaluation methods are needed. PET can serve as a valuable tool to predict LARC response to neoadjuvant therapy, aiding clinicians in post-therapy treatment planning. In a study with 52 LARC patients who received NACRT followed by surgery, PET-CT scans taken 1 week before and 8 to 9 weeks after NACRT were analyzed. Imaging parameters such as post-SUVmax and response index (RI), indicating changes in 18F-FDG uptake, were instrumental in predicting neoadjuvant response and guiding treatment decisions. This is particularly important for patients who achieve a complete clinical response to neoadjuvant therapy and are managed non-operatively.
PET/computed tomography in anal cancers
Role in Disease Staging/Evaluation
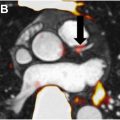
RT is the cornerstone of the treatment of anal cancer with definitive CRT as the treatment of choice in most cases. , NCCN (National Comprehensive Cancer Network) recommends PET/CT in initial staging of anal cancer along with standard CT and MR imaging of the pelvis. The high sensitivity of FDG PET/CT for detecting both primary tumors and nodal involvement surpasses that of CT alone, making it particularly valuable in the initial radiation therapy planning. The ability of PET/CT to detect metabolically active tumor regions that might be missed on CT alone reduces the risk of geographic misses—an outcome where parts of the tumor are not adequately covered by the radiation field. Winton and colleagues showed that PET/CT altered radiation treatment planning in 13% of patients with anal cancer by identifying additional nodal involvement that was not detected by CT. Another systematic review by Albertsson and colleagues showed that with PET/CT, there was a change in target volume delineation in 23% of anal cancer cases. This highlights the importance of PET/CT in ensuring comprehensive coverage of all disease sites during radiation therapy, thereby improving the chances of local control and reducing the risk of recurrence. Fig. 2 shows images of patient with anal cancer who had a small LN in lower para-aortic/common-iliac region which was avid on PET and led to change in treatment volumes to include the para-aortic/common-iliac region.