Vascular Access and Catheter-Directed Angiography
Khashayar Farsad
Frederick S. Keller
Krishna Kandarpa
Indications
Catheter-based diagnostic angiography is most commonly performed either when there is intention to proceed to endovascular intervention or when computed tomography (CTA) or magnetic resonance angiography (MRA) is nondiagnostic or not possible. Indications include:
1. As part of percutaneous endovascular procedures (e.g., thrombolysis, balloon angioplasty, atherectomy, thrombectomy, stenting, embolization, infusion of pharmaceuticals)
2. Diagnosis of primary vascular disease (e.g., vascular occlusive disease, vasculitis, vasospastic disorders, aneurysms, arteriovenous [AV] malformations, AV fistulas)
3. Vascular complications of trauma, surgery, or disease
4. Preprocedural definition of vascular anatomy (e.g., for revascularization procedures, local tumor resection, organ transplantation, complex embolization, assessment of arterial hemorrhage)
5. Diagnosis and localization of vascular tumors (e.g., parathyroid adenomas, pancreatic neuroendocrine tumors)
Contraindications
Absolute
Medically unstable patient with multisystem dysfunction (If angiography is absolutely necessary, underlying abnormalities should be corrected and preventive measures against anticipated complications should be taken.)
Relative
1. Recent myocardial infarction, serious arrhythmia, and substantial serum electrolyte imbalance
2. Serious documented past contrast reaction (see Chapter 64)
3. Impaired renal status (see Chapter 65). Consider prehydration or CO2 angiography.
4. Uncooperative patient (consider general anesthesia)
5. Coagulopathies or seriously altered coagulation profile
6. Inability to lie flat on angiography table due to congestive heart failure or compromised respiratory status
7. Residual barium in abdomen from recent examination (will obscure details of visceral angiography)
8. Pregnancy, because of risk of exposure of fetus to ionizing radiation
9. Ehlers-Danlos syndrome (high risk of arterial injury, dissection)
Preprocedure Preparation
1. Evaluate and document the patient’s chief complaint, brief medical history, significant past medical history, allergies, previous surgical procedures, and
current medications (Fig. 1.1). Explain the procedure to the patient and perform a targeted physical examination including peripheral pulses. All prior imaging studies and physiologic tests (e.g., noninvasive vascular tests, magnetic resonance angiograms, computed tomograms, and radionuclide scans) should be available for review at the time of the study.
current medications (Fig. 1.1). Explain the procedure to the patient and perform a targeted physical examination including peripheral pulses. All prior imaging studies and physiologic tests (e.g., noninvasive vascular tests, magnetic resonance angiograms, computed tomograms, and radionuclide scans) should be available for review at the time of the study.
2. Obtain informed consent (see Chapter e-95).
3. Check laboratory results including estimated glomerular filtration rate (eGFR), hemoglobin, international normalized ratio (INR), partial thromboplastin time (PTT), and platelet count. Routine evaluation of coagulation parameters prior to transfemoral angiography may not be needed in everyone, and limiting evaluation of the coagulation profile to patients who have clinical evidence of a bleeding disorder or liver disease and to those who are anticoagulated may avoid unnecessary testing and delay.
4. Preprocedure hospital fasting guidelines should be followed due to the possible need to administer conscious sedation (e.g., solids and nonclear liquids 6 to 8 hours, clear liquids 2 to 4 hours) (1). Oral medications may be taken with small quantities of water.
5. See Chapter 65 for a detailed approach to the hydration of a patient with underlying renal disease.
6. Patient must void urine before leaving for the angiography suite (unless the bladder is catheterized).
7. Considerations for patients with specific diseases or conditions: Consult with referring or managing clinician, or both, on all items listed below (for more specific details, see the appropriate cited chapters).
a. Heparinized patient: Stop heparin infusion 2 hours prior to the arterial puncture in order to normalize the coagulation status. A PTT of 1.2 times control is acceptable, in the absence of other bleeding abnormalities. Alternatively, activated clotting time (ACT) may be followed. Because this test can be performed at the bedside, the timing of catheter removal and reinstitution of heparin can be more accurately determined. Heparin may be restarted within 2 to 4 hours after removal of the catheter for manual puncture-site compression or sooner in selected cases (e.g., patients for whom an arterial puncture closure device was used or venous catheterizations). For patients getting therapeutic dose injections of low-molecular-weight heparin, the dose prior to the procedure is held (2).
b. Warfarinized patient: Stop warfarin (Coumadin) 3 to 5 days prior to arterial puncture if possible. For hospitalized patients with persistently elevated INR and nonurgent indications for angiography, vitamin K (2 to 10 mg) may be administered either orally 24 to 48 hours preprocedure or intravenously (IV) 12 to 24 hours preprocedure with serial monitoring of the INR for reversal (3). Patients who require procedures on an urgent or emergent basis should be treated with short-acting products to reverse anticoagulation, such as fresh frozen plasma (FFP), prothrombin complex concentrate (PCC), recombinant factor VIIa, or activated PCC. The goal is to achieve an INR of 1.5 or less. For patients in whom discontinuation of anticoagulation is unacceptable (e.g., metal prosthetic heart valve), warfarin can be stopped and anticoagulation may be transitioned with low-molecular-weight heparin as an outpatient, or the patient may be admitted for transition with IV heparin.
c. Oral anticoagulants: Three newer oral anticoagulant medications include the direct thrombin inhibitor, dabigatran, and two direct factor Xa inhibitors, rivaroxaban and apixaban. These medicines have relatively short halflives (<14 hours). As such, discontinuation for 1 to 2 days prior to arterial puncture is usually sufficient (longer in patients with renal disease because these drugs are cleared by the kidneys) (4,5). Recently, a monoclonal antibody fragment, idarucizumab, has been approved by the U.S. Food and Drug Administration to neutralize the effects of dabigatran in the event of a bleeding emergency.
d. Antiplatelet agents: These include aspirin, clopidogrel, and glycoprotein (GP) IIb/IIIa inhibitors. Guidelines recommend discontinuation of the latter two until cleared (5 to 7 days for clopidogrel, 8 to 48 hours for GP IIb/IIIa inhibitors) or potential platelet transfusion for emergency cases (2,5).
e. Thrombocytopenic patient: For transfemoral or transaxillary punctures, the functional platelet count should be greater than 50,000 per µL (2).
f. Insulin-dependent diabetic patient: In consultation with the referring physician, cut the morning insulin dose by half and schedule the procedure for the morning if possible. A slow infusion of 5% dextrose may be started prior to the procedure, and volume expansion with IV fluids is indicated to prevent contrast-induced nephropathy. Blood glucose levels should be monitored during prolonged procedures; insulin dose may need to be titrated before
resumption of usual regimen. If a diabetic patient on neutral protamine Hagedorn (NPH) insulin receives heparin during the procedure, do not reverse the heparin with protamine sulfate because this may cause a fatal anaphylactic reaction (6). Restart normal insulin schedule after the procedure once the patient has resumed oral intake.
resumption of usual regimen. If a diabetic patient on neutral protamine Hagedorn (NPH) insulin receives heparin during the procedure, do not reverse the heparin with protamine sulfate because this may cause a fatal anaphylactic reaction (6). Restart normal insulin schedule after the procedure once the patient has resumed oral intake.
g. Renal dysfunction (see Chapter 65)
h. Prior documented reaction to iodinated contrast media: Use measures described in Chapters 64 and e-89. Alternatively, consider gadolinium-enhanced MRA for patients without severe renal insufficiency. Carbon dioxide arteriography, intra-arterial pressure measurements, and duplex scan (infrainguinal) arterial mapping may aid diagnosis in selected patients. (See appropriate chapters.)
i. Precautionary measures: The patient’s chart should list the precautions necessary to protect both the patient (especially if immunocompromised) and the personnel who may come in contact with a patient with an infectious disease (e.g., HIV, infectious hepatitis, drug-resistant bacteria, Clostridium difficile).
j. Lidocaine hypersensitivity (local infiltration) (see Chapter 62). Consider:
(1) Local skin test, and if negative, proceed with local infiltration, or
(2) Procaine hydrochloride (or an aminoester local anesthetic rather than an aminoamide)
a. Sedation and analgesia: Most angiography and interventional procedures can be completed safely and expeditiously with a judicious combination of midazolam and fentanyl, which provide adequate conscious sedation and analgesia (7).
b. Age: Reduce medication doses by 30% to 50% for elderly patients.
c. Severe coronary artery or cerebrovascular disease: Avoid drugs that cause excessive drop in blood pressure or cardiac output.
d. Seizures: Avoid drugs that lower seizure threshold (e.g., meperidine, phenothiazines).
e. Hepatic dysfunction: Avoid drugs such as barbiturates, which are metabolized by the liver. Reduce initial doses of sedatives and analgesics.
f. Renal dysfunction: Extreme caution should be used in administering meperidine. Accumulation of its metabolite in these patients may lead to central nervous system excitation and seizures.
g. Pheochromocytoma: Patients with labile blood pressure need α blockade (e.g., phenoxybenzamine) (8). Consider consulting an anesthesiologist for the procedure. Short-acting agents, such as sodium nitroprusside, should be available for treating potential hypertensive crisis. Avoid the use of glucagon in patients with suspected pheochromocytoma.
h. Multiple myeloma: As with patients with diabetic nephropathy, these patients should be well hydrated in order to prevent acute tubular necrosis.
i. Sickle cell anemia and polycythemia vera: Patients may suffer thromboembolic complications following angiography (9).
Procedure
Retrograde Femoral Artery Catheterization, Seldinger Technique
1. Preparation
a. Sterile puncture-site preparation (iodine or chlorhexidine solution scrub following groin shave) and draping of patient. The patient must be in a comfortable position that can be tolerated for the duration of the procedure prior to preparation.
b. All patients subjected to any angiographic or interventional procedure under conscious sedation should have continuous physiologic monitoring (see Chapters 62 and 63).
c. Induce local anesthesia with 1% or 2% lidocaine (without epinephrine). Consider the addition of 1 mL sodium bicarbonate 8.4% in the syringe with each 10 mL of lidocaine to minimize the burning sensation during injection.
(1) Skin wheal at the entry site (using 25-gauge, 5/8-in. needle) and deep on each side of the artery in an inverted cone distribution (using 22- to 25-gauge, 1.5-in. needle).
(2) Avoid entering the artery or vein and injecting lidocaine into the vessel wall by gentle aspiration as the needle is advanced, and injection of anesthetic upon needle withdrawal. Slow, gentle injection will save the patient considerable discomfort. Wait 1 to 2 minutes after injection before making a superficial skin incision (3 mm long × 3 mm deep) with a no. 11 blade scalpel.
(3) Use a curved 5-in. mosquito forceps to spread the subcutaneous tissues; avoid spreading down to the artery. Adequate dissection of subcutaneous tissues in this manner facilitates subsequent passage of catheters and sheaths, enables egress of blood at the skin rather than internally, and is also important when considering use of an arterial closure device to allow the device to track easily to the arteriotomy site.
d. Check that fluoroscopy is working before the artery is punctured.
2. Femoral artery puncture (Fig. 1.2)
a. Locate the femoral artery and inguinal ligament (which runs from the anterior superior iliac spine to the pubic tubercle) by palpation (Fig. 1.3). Th e true position of the inguinal ligament is about 1 to 2 cm below the location estimated by palpation or fluoroscopy (11).
b. Localizing the puncture site by fluoroscopy over the femoral head or with specific palpation techniques for anatomical localization is important to
(1) Prevent high arterial entry that cannot be adequately compressed and may lead to uncontrollable internal bleeding
(2) Prevent low arterial entry that may result in pseudoaneurysm of the superficial femoral artery
c. The artery should be entered over the middle of the medial third of the femoral head; the skin entry site should be over the inferior femoral head so that the arterial puncture is directly over the femoral head after the needle has passed through the subcutaneous tissues. This puncture location enables adequate compression of the artery against the femoral head at the end of the procedure. A window of only 3 to 5 cm is available for safe common femoral artery puncture (Fig. 1.4).
d. Ultrasound guidance: Routine use has demonstrated decreased frequency of access site-related complications in large retrospective and prospective series (12,13) and should be considered for all punctures. This technique allows precise identification of the common femoral artery and allows for direct single-pass puncture. Ultrasound guidance is ideal for patients with poorly palpable pulses and patients who are obese or who have higher bleeding risks. The technique is as follows (Fig. 1.5):
(1) Localize the femoral head under fluoroscopy as described earlier.
(2) Use a high-frequency linear transducer draped with a sterile probe cover.
(3) Identify the common femoral artery and vein. The common femoral vein is identified by the confluence with the great saphenous vein and will be easily compressible in the absence of thrombus. The common femoral artery is directly lateral to the common femoral vein and will be pulsatile and not easily compressible. The common femoral artery bifurcation should be identified by grayscale or color Doppler to confirm a common femoral artery puncture above the bifurcation. Acoustic shadowing from calcified plaque may obscure the artery lumen. Color
Doppler can be used in this instance to confirm patency and target for puncture if needed.
Doppler can be used in this instance to confirm patency and target for puncture if needed.
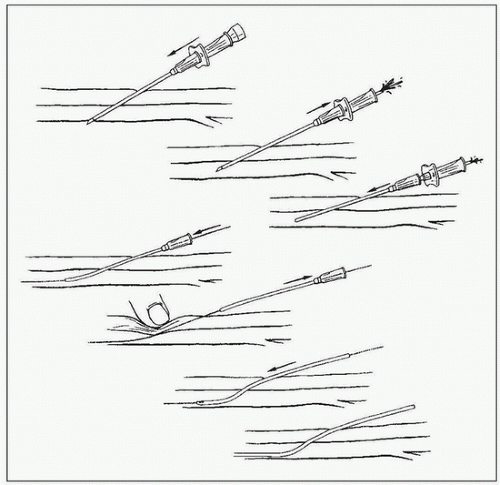 FIGURE 1.2 • Schematic of the Seldinger puncture technique using a needle covered by a plastic sheath. Top to bottom: (1) Needle and stylet are introduced as a unit into the artery; (2) stylet is removed and needle is withdrawn until brisk pulsatile backflow of blood is noted; (3) inner metallic cannula is removed; (4) a wire is introduced through the plastic sheath; (5) wire is fixed and sheath is removed with compression over the puncture site; (6
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|