Vena Caval Filters
Jennifer P. Montgomery
John A. Kaufman
Vena caval filters are intravascular devices designed to prevent pulmonary embolus (PE) by trapping venous emboli. Filters do not prevent formation of new thrombus or promote lysis of a preexisting thrombus or embolus. The primary means of therapy and prophylaxis for deep vein thrombosis (DVT) and PE are pharmacologic.
There are three basic classes of filters.
1. Permanent filters (1): Permanent vena caval filters are devices that are not intended to be repositioned or retrieved in any manner. These are the oldest class of filters, for which the greatest experience is available.
2. Optional filters (2): Permanent filters designed to provide the option of percutaneous removal or conversion to a nonfiltration state. The two basic types are retrievable and convertible filters.
a. Retrievable filters can be retrieved or repositioned percutaneously during a device-specific time window, after which they become incorporated into the wall of the vena cava and function as permanent devices. Manufacturers suggest ranges of retrievability on the basis of clinical trials and experience. In practice, filter retrievability over time may vary. Retrieval of these devices is not required.
b. Convertible filters can be altered structurally after implantation so that they no longer function as filters. After conversion, some or all of the filter remains in the patient’s vena cava without providing protection from PE. When conversion is by mechanical means and requires a percutaneous procedure, conversion of these devices is not required, and the filter can provide permanent protection. When conversion is built into the filter so that it occurs without intervention, then the duration of protection from PE is limited.
3. Temporary filters: Temporary filters are not designed for permanent placement. They frequently have no means for fixation to the wall of the vena cava but are supported in place by tethers or catheters that either exit the skin at the insertion site or are buried subcutaneously. Removal of these devices is required, and permanent filtration requires removal of the temporary filter and placement of a different device. The indications for placement of temporary filters are the same as those for retrievable filters.
Filter Placement
Indications
There remains significant controversy over the appropriate indications for vena cava filter placement among professional medical societies (3).
1. Accepted: documented venous thromboembolism (VTE) with one or more of the following:
a. A contraindication to anticoagulation, such as active gastrointestinal bleeding (not fecal occult blood), recent intracranial hemorrhage or surgery, or vascular brain metastases
b. Documented progression or recurrence of VTE while anticoagulated. The definition of adequate anticoagulation is vague but is generally accepted to be 7 days of continuous anticoagulation at therapeutic levels.
c. A complication of anticoagulation, such as massive retroperitoneal hemorrhage, that requires interruption or termination of anticoagulation
d. Inability to achieve or maintain therapeutic anticoagulation
2. Relative (evaluated on a case-by-case basis): documented VTE with one or more of the following:
a. Limited cardiac or pulmonary reserve
b. Massive, life-threatening PE that requires thrombolysis or surgical thrombectomy
c. Chronic PE treated with thromboendarterectomy
d. Poor patient compliance with medications, risk of falling, or inability to monitor patient during treatment
e. Large residual burden of thrombus (iliocaval DVT), or “widow-maker” thrombus in the inferior vena cava (IVC)
f. Recurrent PE in spite of the presence of an existing vena caval filter (failure to trap embolus or propagation of thrombus above filter) and continued contraindication to anticoagulation
g. Difficulty establishing therapeutic anticoagulation
h. Thrombolysis for iliocaval DVT
3. Prophylactic (evaluated on a case-by-case basis): no documented current VTE and
a. Severe trauma patient with high risk of VTE
b. Closed head injury
c. Spinal cord injury
d. Multiple long bone or pelvic fractures
e. High-risk patients with prolonged immobilization
Optional and Temporary Filters
1. The same as earlier, when the anticipated need for the filter will end within the time limitations during which the filter can be retrieved or converted
Contraindications
1. There are rare circumstances in which filters may be contraindicated, including
a. Total thrombosis of the vena cava
b. Inability to gain access to the vena cava
c. Inability to image during filter placement
d. Vena cava too small or too big to safely accommodate the filter
e. Confirmed allergy to a component of the filter
2. The following are not contraindications to filter placement:
a. Ongoing sepsis (this includes septic thrombophlebitis, as the alternative to trapping infected thrombus in a filter is septic pulmonary emboli)
b. Inability to document residual peripheral thrombus in a patient with PE and an indication for a vena caval filter. Current imaging techniques cannot fully evaluate all possible sources of emboli, and more thrombus may form and embolize after imaging.
Preprocedure Preparation
1. The consent for the placement of a vena caval filter should include all of the usual risks associated with percutaneous venous procedures as well as the following (6):
a. A 5% risk of recurrent PE
b. A 1% to 5% risk of symptomatic caval thrombosis (remember, the intended purpose of the filter is to trap emboli)
c. A 1% risk of filter embolization, fracture, or malposition
d. A less than 1% risk of symptomatic perforation by a filter element
2. Laboratory values (suggested guidelines—actual practice may vary)
a. International normalized ratio (INR) less than 3.0
b. Platelets greater than 30,000 per µL
3. Patient evaluation
a. Review available cross-sectional imaging of the abdomen for anomalous caval anatomy and presence of thrombus.
b. Assess for availability of venous access (trauma patients with neck braces, pelvic fixation, existing lines, etc.).
Procedure
1. Obtain access: Depending on the device, vena caval filters can be placed from the femoral veins, jugular veins (internal or external), subclavian veins, upper extremity veins, or directly into the IVC via translumbar approach. The preferred approach for filters that are not delivered over a guidewire, or filters of a relatively rigid design, is either the right femoral or internal jugular vein.
2. Imaging: High-quality imaging during filter placement maximizes the likelihood of a satisfactory outcome. Poor imaging increases the chance of filter misplacement and other operator errors (8,9).
a. Goals of preplacement imaging
(1) Define vena caval and renal vein anatomy.
(a) Duplicated IVC occurs in less than 1% and usually joins at the left renal vein but may also join lower.
(b) Left-sided IVC is found in less than 1%.
(c) Circumaortic left renal vein is found in 3% to 4%. The lower component of the venous ring lies behind the aorta and drains into the IVC lower than a normal left renal vein.
(d) Retroaortic left renal vein occurs in 2% to 3% and usually drains into the IVC below the right renal vein or rarely at the confluence of the iliac veins.
(e) Duplicated SVC occurs in less than 1%.
(f) Persistent left SVC drains into the coronary sinus.
(g) Left-sided SVC is extremely rare.
(2) Determine vena cava size.
(a) “Mega cava” (IVC diameter >28 mm) is found in less than 1%.
(b) IVC is typically oval in cross-section, so measurements in a single plane may not be accurate.
(3) Confirm patency of vena cava.
b. Cavography
(1) For IVC, position pigtail catheter (4 Fr. or greater) at the confluence of the iliac veins.
(2) For SVC, inject from a brachiocephalic vein.
(3) Use the same positioning and field of view that you will employ during filter deployment.
(4) Injection rate for iodinated contrast of 15 to 20 mL per second for 2 seconds.
(5) CO2 cavography can be performed by hand injection of 30 to 40 mL CO2.
(6) Digital subtraction angiography (DSA) filming at four to six frames per second during suspended respiration in anterior-posterior projection. Additional projections can be obtained as needed. Contralateral iliac vein and renal veins are identified as inflow of unopacified blood or by reflux of contrast into orifices of veins.
(7) If veins cannot be localized, try the following: Reposition pigtail catheter closer to the expected location of tributaries, increase rate and volume of contrast injection, use oblique projection, selectively catheterize veins, and use intravascular ultrasound (IVUS).
c. IVUS: Alone or combined with fluoroscopy, IVUS has been used to guide filter placement in patients with contraindications to all contrast agents and for bedside filter placement (10,11).
(1) Femoral venous access is easiest when using IVUS alone (avoids negotiating right atrium).
(a) Tandem ipsilateral access, bilateral access, or single puncture technique
(2) Determine IVC dimensions and patency.
(3) Localize renal vein orifices and iliac confluence.
(a) Note locations with fluoroscopy or measure distance from venous access site.
(4) Deploy filter using fluoroscopic guidance if available.
(5) Obtain plain radiograph of the abdomen to document filter position and configuration.
d. Ultrasound: Transabdominal ultrasound (US) guidance for filter placement, usually at the bedside, has been described (12). This approach has the following limitations:
(1) The IVC is not always easy to image in large patients or those with significant bowel gas.
(2) Variant renal vein and IVC anatomy may not be detected.
3. Filter placement
a. General principles
(1) After performing initial imaging, note the level of renal vein inflow and confluence of iliac veins relative to a fixed reference point, such as the spine, a radiopaque ruler, or other measuring device.
(2) Without moving the patient or image intensifier, exchange the pigtail catheter over a guidewire for a filter delivery sheath.
(3) Watch under fluoroscopy when reinserting the guidewire into the pigtail catheter to avoid malpositioning the guidewire (e.g., into an ascending lumbar vein).
(4) Serial dilation may be required for large delivery systems. A stiff or exchange-length guidewire may be needed for difficult or remote access.
(5) Position the delivery sheath central to the desired final location of the filter when placing from the femoral approach; position the sheath peripheral to the renal veins when access is from above.
(6) Leave guidewire in place for over-the-wire delivery system (make sure to use a straight guidewire to avoid guidewire entanglement in the filter post deployment).
(7) Before advancing filter into delivery sheath, inspect to be sure that the orientation of the filter is correct for the chosen access route (i.e., femoral or jugular).
(8) Advance filter to the end of the delivery sheath. Reposition the entire system so that the constrained filter is in the desired location.
(9) Deploy the filter per manufacturer’s instructions.
(10) Withdraw the delivery sheath several centimeters below the filter when access is from the groin; leave the sheath at the top of the filter when deployed from above.
(11) Repeat cavogram through the delivery sheath using the same injection and filming rate as described earlier.
b. Filter location (Fig. 35.1)
(1) Normal IVC
(a) The top of a single-level cone-shaped filter should be just at or slightly above the lower edge of the orifice of the lowest renal vein. This minimizes potential “dead space” above the filter should filter occlusion occur.
(b) The top of a bi-level cone-shaped filter (a device with upper arms and lower legs) should be below the orifice of the lowest renal vein to prevent prolapse of one of the arms into a renal vein.
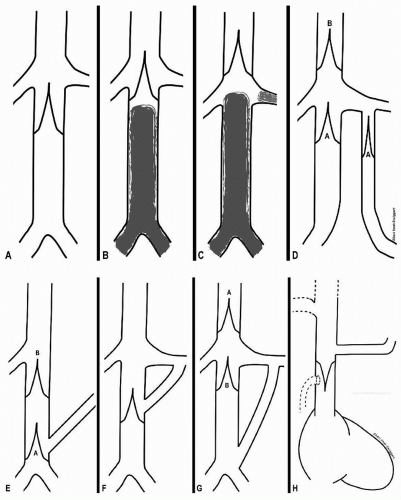 FIGURE 35.1 • Suggested VC filter placement locations for (A) a normal infrarenal IVC with no intraluminal thrombus; (B) infrarenal IVC thrombus, not quite approaching renal veins and with adequate room for filter legs to attach; (C) infrarenal and renal vein thrombus; (D) duplicated IVC—either two infrarenal filters (A) or a single filter (B) in suprarenal segment; (E) retroaortic (low insertion) of a left renal vein (A or B); (F) circumaortic left renal vein; (G) left renal vein insertion at confluence of left iliac vein and IVC (A or B); (H) SVC placement to confine upper extremity emboli. |
(c) Filters that are not cone-shaped should be placed below the renal vein orifices.
(d) All filters should be placed so that there is adequate wall contact between the stabilizing filter elements and the IVC.
(e) When a filter is already present and does not contain thrombus and the indication is recurrent PE, a second filter can be placed below the first if sufficient room is present. Otherwise, place a second filter above the first (including suprarenal if necessary).
(2) Thrombus in IVC
(a) Thrombus does not extend to the renal veins: Place the filter as low as possible in the infrarenal IVC but above thrombus, even if the body of the filter lies entirely within the intrarenal IVC. If there is little room for infrarenal attachment of the filter, consider a cone-shaped device or a suprarenal placement.
(b) Thrombus extends to or originates from the renal veins: Place the filter in the suprarenal segment, either just above the renal veins or in the intrahepatic IVC. Use a short filter (i.e., not Bird’s Nest, as wires may prolapse into the right atrium and cause arrhythmia).
(c) Thrombus extends above the existing filter: Place a second filter above thrombus, usually in the suprarenal IVC.







