Venous compressive disorders are a heterogenous group of vascular syndromes characterized by extrinsic venous compression that can lead to complications of venous hypertension or venous thrombosis. Endovascular damage secondary to deep venous thrombosis (DVT) can result in post-thrombotic syndrome (PTS), a potentially debilitating condition that can be associated with significant morbidity in the pediatric population. Here we discuss 4 venous compressive disorders: iliac vein compression (May-Thurner syndrome [MTS]); subclavian vein compression at the venous thoracic inlet (Paget-Schroetter syndrome); left renal vein compression (nutcracker syndrome); and popliteal vein compression (popliteal entrapment syndrome) with a focus on clinical evaluation and diagnostic methods. Where endovascular therapy is appropriate, specific procedural considerations including procedure indications, equipment, procedural steps, technical challenges, complications, clinical follow-up and expected outcomes are discussed.
Iliac vein compression (May-Thurner syndrome)
Background and clinical evaluation
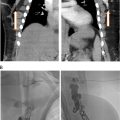
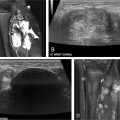
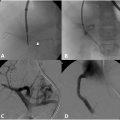
May-Thurner syndrome is characterized by extrinsic compression typically by the right common iliac artery on the left common iliac vein against the fifth lumbar vertebra ( Fig. 1A ), and can result in compressive nonthrombotic iliac vein lesion (NIVL) or venous stasis leading to secondary ipsilateral acute or chronic deep vein thrombosis (DVT). May and Thurner theorized that chronic arterial pulsations and mechanical compression result in endothelial proliferation and development of venous intimal hyperplasia in the form of webs and spurs ( Fig. 1B ). It has been postulated that repetitive microtrauma leading to endothelial injury along with hemodynamic alterations of the vessel wall may contribute to downstream venous thrombosis particularly in conditions of hypercoagulable states. , Retrospective studies have shown that patients with compression of the left iliac vein to a 4 mm diameter, as measured on CT venography, have a 4-5× fold increased risk of acute DVT. It has further been demonstrated that this anatomy contributes to 90% of oral-contraceptive induced or pregnancy induced DVTs affecting the left limb. Endovascular damage related to DVT can lead to significant long-term morbidity when it is associated with the development of post thrombotic syndrome (PTS), a chronic condition characterized by venous insufficiency that includes symptoms such as limb swelling, pain, discoloration, and skin ulceration of the affected limb. Initial evaluation when the diagnosis of iliac vein compression resulting in lower extremity DVT is considered is duplex ultrasound ( Fig. 2A – C ). Transabdominal ultrasound can be used in the pediatric population to assess the IVC, left renal vein, and iliac veins however depending on body habitus and technical factors, adequate visualization of the IVC and iliac system can be challenging. If iliocaval venous outflow obstruction is suspected or identified on transabdominal ultrasound, venogram can be performed to confirm the diagnosis and establish a treatment plan. Noninvasive imaging modalities such as CT or MRI are often utilized if ultrasound is inconclusive and to assess vein size. CT venography (CTV) can detect iliac vein stenosis with 95% sensitivity ( Fig. 2D ) whereas MR venography (MRV) is increasingly utilized in the pediatric population to reduce radiation exposure ( Fig. 2E ). MR venography may also improve imaging of iliac and caval thrombosis by reducing variability of contrast enhancement based on timing and, with the use of flow sensitive sequences, allow the evaluation of direction of flow. , ,


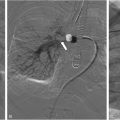
Conventional venography has traditionally been the gold standard imaging in diagnostic confirmation of venous obstruction and to evaluate the degree of luminal stenosis, presence of collateral vessels and post obstructive dilatation and allows treatment to be offered in the same setting ( Fig. 3 ). Hemodynamic pressure gradient measurement has historically been used as a valuable adjunct in confirming significant stenotic lesions with a gradient of >2 mmHg indicating hemodynamic significance. , However, many experienced practitioners are skeptical of this value given how dependent this gradient is on hydration status, patient position, and vasomotor tone, as unlike the arterial system, the venous system is dependent on these factors to affect size. In some studies, intravascular ultrasound (IVUS) imaging has demonstrated superiority over conventional single view venography in predicting when stenting iliofemoral vein stenosis results in significant symptom improvement and has been utilized in formulating a treatment plan . However, diligent 2 view contrast venography may also be used to determine the degree of obstruction; including the degree of stenosis, and importantly, the presence of collaterals, but is associated with an increased radiation dose to the patient.

Procedural indications and technique
Nonthrombotic iliac vein lesion
Noninvasive imaging may demonstrate iliac vein compression however, in the absence of a history of DVT, a patient must have lifestyle- limiting swelling and/or pain before proceeding with an invasive procedure. In addition, other causes of these symptoms (lymphedema, superficial or deep venous reflux, or neurogenic issues) must be ruled out prior to proceeding with intervention. Furthermore, the patient must be counseled that if a stent is placed that there may be only mild or no improvement in symptoms. Subsequently, conservative treatments were preferred.
If a stent is placed it is typically for an ipsilateral common femoral vein access. Since the normal diameter of the iliac vein is 12.2 mm, stents most commonly used are 12-16 mm in diameter and 80 mm in length. Diameters larger than this may be a misrepresentation of the actual vein size, especially if IVUS is not utilized. The AP diameter of the vein may be up to 22 mm, with the lateral view measuring only 2-3 mm. When a large diameter stent is placed, the patient may be at increased risk of back pain due to irritation of the lumbar periosteum.
Thrombotic lesion
DVT has been historically treated with anticoagulation and compression stockings, although compliance with compression is limited. When DVT is present, systemic anticoagulation with compression stockings were historically standard therapy however, early endovascular therapy with lytic therapy and treatment of the venous compression with stent is currently advised in iliofemoral DVT, when the patient has a low risk of bleeding, and the institution is skilled in endovascular treatment of DVT. Endovascular therapy can include catheter-directed thrombolysis with pharmacological agents or various mechanical thrombectomy devices. Early thrombolysis can preserve in-line outflow and valve function and prevent or decrease the incidence of post-thrombotic syndrome. The underlying contributor to DVT, the venous compression, is simultaneously treated with stenting. There are multiple FDA self-expanding stents now approved for venous stenting.
In acute iliofemoral venous thrombosis secondary to May-Thurner, catheter-directed thrombolysis (“infusion-first” or “single-session”), angioplasty and stent placement offer symptomatic relief and good long-term patency. Prophylactic IVC filters are not typically placed unless the consequence of even small PE is great, a large free-floating iliac or IVC clot is found, or mechanical thrombectomy without enzymatic lysis is warranted because of excessive bleeding risk.
Ultrasound guided percutaneous access is achieved with a micro-puncture set commonly at the popliteal vein or, depending on the extent of thrombus, occasionally the posterior tibial vein. Choosing the latter as an access vessel should take into consideration expected sheath sizes. Ideally, access is performed in a patent vein if attempting single session treatment, as the goal of therapy is flowing vein to flowing vein. Access via the internal jugular vein or femoral veins have also been reported. It has been the authors’ experience that single session treatment is successful if the age of the clot is <14 days, but ideally <7 days. However, the recent advent of much more powerful mechanical thrombectomy devices some patients can be treated with clot older than 14 days. In the event that all veins in the lower extremity are thrombosed, single session is not recommended. Instead, the protocol as used in the ATTRACT trial is common practice at the authors’ institution which includes popliteal vein access and placement of a 6 Fr sheath. The venous occlusion is then traversed with hydrophilic coated wires and catheters and conventional hand injection venography is performed to define the thrombus extent, followed by thrombolysis with a multislit side hole catheter. In two 1000 cc normal saline bags, 10 milligrams of alteplase (Genentech, San Francisco) are placed into each bag for a concentration of 0.01 mg/cc. One bag is connected to the multislit catheter and the other through the side arm of the sheath. Total dose is not to exceed 1 mg/hr, or 100 cc/hr. In addition, heparin is administered at 400u/hr, and the partial thromboplastin time is monitored not to exceed 60-80 seconds. Heparin assay or Anti Xa level can also be monitored. The therapeutic range is 0.3-0.5 IU/ml during a lytic infusion, which is slightly lower than the normal therapeutic range of 0.3-0.7 IU/ml. It is important to remember that if mechanical thrombectomy is used, hemolysis may affect the results of all of these values. Typical infusion duration for lytic therapy is 20-30 hours in an ICU or step-down unit to monitor for bleeding. Mechanical thrombectomy is often performed on the second day, with upsizing of the sheath to allow larger thrombectomy devices and stent placement. A number of mechanical venous thrombectomy devices are available and include: AngioJet, Penumbra Cat 12, JETi, Inari ClotTriever. These devices can be used for single-session treatment or to remove residual thrombus after overnight alteplase infusion. There have been no direct comparisons of these devices and device selection is currently based on provider preference and availability. Data presented the 2023 SIR Annual Meeting suggested a very high rate of popliteal occlusions in patients that were treated with the ClotTriever (Inari Medical) (Goloubev, A., Kang, R., et al. Comparing incidence of recurrent iliofemoral deep venous thrombosis following ClotTriever mechanical thrombectomy and thrombolysis: A multicenter retrospective review. Presented at: SIR 2023 (abstr)).
After 70%-100 % of the thrombus is removed, balloon angioplasty and stent placement may be performed in the treatment of obstructive lesions ( Fig. 3B ). Self-expandable stents with radial flexibility may be used in the iliac vein and extended as low at the lesser trochanter if needed. Self-expanding stents are preferable in the iliac vein. Balloon angioplasty is preferred for femoropopliteal stenotic lesions.
Postprocedure care and clinical follow-up
The most important aspect of postprocedure care is proper anticoagulation management. It is advised to use enoxaparin (1 mg/kg SQ BID), for at least 1 month. Following this, the patient is transitioned to a direct oral anticoagulant (DOAC). Most centers favor apixaban 5mg BID over rivaroxaban 20 mg per day due to a slightly lower bleeding profile and more homogenous blood levels. However, if a patient prefers once a day dosing, rivaroxaban is acceptable. Routine use of anti-platelets has not been proven. Centers with the longest experience do not use them routinely, and if an anti-platelet is use, it is typically 81 mg aspirin per day. The duration of anticoagulation should follow the recommendations of the American College of Chest Physicians, regarding provoked vs. nonprovoked DVT. The efficacy of compression stockings has not been proven but may provide individual patients relief. If the patient has residual swelling after intervention either calf high or thigh high compression stockings at 20-30 mmHg can be tried. Higher compression can be utilized if these do not improve the symptoms, however, if significant swelling is persistent, then other mechanical or physiologic causes should be sought.
Clinical follow-up is recommended to ensure compliance with anticoagulation and to ensure resolution of symptoms. Most practitioners see patients in follow-up at 1 month, 3 months, 6 months, and then every 12 months. Some practices routinely obtain duplex ultrasounds at follow-up. At the authors’ institution imaging is not typically obtained unless there is leg swelling and/or pain. It is recommended to obtain imaging prior to cessation of anticoagulation, and depending on body habitus and stent location this can either be CT venogram or duplex ultrasound.
Expected outcomes
Primary patency rates from prospective FDA approval trials for iliofemoral venous stents range from 85%-90%. In the largest and most recent long-term retrospective study primary, primary assisted, and secondary patency rates at 5 years were 57%, 77%, and 80% by Kaplan Meier methods and 79%, 90%, and 93% by summary statistics methods. Of note, this cohort included 40% with iliocaval stents, a more severe disease, and 60% with iliofemoral stents.
Subclavian vein compression (Paget-Schroetter syndrome)
Background and clinical evaluation
Paget Schroetter Syndrome (PSS) refers to a specific variant of thoracic outlet syndrome caused by anatomic compression of the subclavian vein at the level of the thoracic outlet resulting in microvascular trauma and DVT. The costoclavicular triangle is formed medially by the costoclavicular ligament, posterolaterally by the insertion of the anterior scalene muscle, the first rib inferiorly and the medial aspect of the clavicle superiorly. PSS primarily occurs in the dominant arm and is classically described as “effort thrombosis” due to pathophysiology of excessive physical activity, particularly repetitive overhead motions, causing extrinsic compression of the subclavian vein in the costoclavicular space. Compression of the subclavian vein at the costoclavicular space can also occur secondary to first rib or clavicle post traumatic deformities, hypertrophy of adjacent ligaments or tendons and rarely other space occupying masses or the presence of anatomic abnormalities such as a cervical rib.
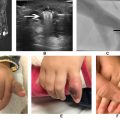
Clinical symptoms of unilateral arm pain, swelling and fatigue may be secondary to venous hypertension from acute or chronic venous obstruction and valvular reflux secondary to inflammation and fibrosis damaging valves. Hand cyanosis as well as enlarged collateral vessels in the shoulder and chest wall may also be noted, known as Urschel’s sign. PSS is estimated to occur in approximately 2-11 out of 100,000 people. Positional compression is exacerbated by prolonged shoulder abduction. At-risk individuals include athletic individuals such as swimmers, weight lifters, pitchers and rowers. Patients with acute upper-extremity deep vein thrombosis due to subclavian vein compression ( Fig. 4A ) are at risk for pulmonary emboli. Rarely, unrelenting significant venous outflow obstruction of both the superficial and deep systems can cause downstream arterial and capillary compromise resulting in limb threatening phlegmasia cerulea dolens.