Recent advances in pediatric neurosurgery have drastically improved the outcome in infants and children afflicted with surgical lesions of the central nervous system (CNS). Because most of these techniques were first applied to adults, the physiologic and developmental differences that are inherent in pediatric patients present challenges to neurosurgeons and anesthesiologists alike. The aim of this paper is to highlight these age-dependent approaches to the pediatric neurosurgical patient.
Recent advances in pediatric neurosurgery have dramatically improved the outcome in infants and children afflicted with surgical lesions of the central nervous system (CNS). Although most of these techniques were first applied to adults, the physiologic and developmental differences that are inherent in pediatric patients present challenges to neurosurgeons and anesthesiologists alike. The aim of this paper is to highlight these age-dependent approaches to the pediatric neurosurgical patient.
Developmental considerations
Age-dependent differences in cerebrovascular physiology and cranial bone development influence the approach to the pediatric neurosurgical patient. Cerebral blood flow is coupled tightly to metabolic demand, and both increase proportionally immediately after birth. Estimates from animal studies place the autoregulatory range of blood pressure in a normal newborn between 20 and 60 mmHg . This range is consistent with relatively low cerebral metabolic requirements and low blood pressure during the perinatal period. More importantly, the slope of the autoregulatory slope drops and rises significantly at the lower and upper limits of the curve, respectively. This narrow range, with sudden hypotension and hypertension at either end of the autoregulatory curve, places the neonate at risk for cerebral ischemia and intraventricular hemorrhage, respectively. Another developmental difference between adults and pediatric patients is the larger percentage of cardiac output that is directed to the brain, because the head of the infant and child accounts for a large percentage of the body surface area and blood volume. These factors place the infant at risk for significant hemodynamic instability during neurosurgical procedures.
The infant cranial vault is also in a state of flux. Open fontanels and cranial sutures lead to a compliant intracranial space. The mass effect of a tumor or hemorrhage are often masked by a compensatory increase in the intracranial volume through the fontanels and sutures. As a result, infants presenting with signs and symptoms of intracranial hypertension have fairly advanced pathology.
Preoperative evaluation and preparation
Closed-claim studies have revealed that neonates and infants are at higher risk for morbidity and mortality than any other age group . Respiratory and cardiac-related events account for a majority of these complications. However, a major pitfall in the management of infants and children for neurosurgery is the presence of coexisting diseases. Given the urgent nature of most pediatric neurosurgical procedures, a thorough preoperative evaluation may be difficult. However, a complete airway examination is essential, because some craniofacial anomalies may require specialized techniques to secure the airway . Most cardiac morbidity due to congenital heart disease occurs during the first year of life . Congenital heart disease may not be apparent immediately after birth, and the hemodynamic alterations caused by anesthetic agents, mechanical ventilation, and blood loss during surgery can unmask these cardiac defects. Echocardiography can be helpful in the assessment of the heart, and a pediatric cardiologist should evaluate patients with suspected problems to help optimize cardiac function prior to surgery. Other coexisting diseases that can alter the conduct of anesthesia are list in Table 1 .
| Condition | Anesthetic implications |
|---|---|
| Congenital heart disease | Hypoxia and cardiovascular collapse |
| Prematurity | Postoperative apnea |
| Upper respiratory tract infection | Laryngospasm and postoperative hypoxia/pneumonia |
| Craniofacial abnormality | Difficulty with airway management |
| Denervation injuries | Hyperkalemia after succinycholine |
| Resistance to nondepolarizing muscle relaxants | |
| Chronic anticonvulsant therapy for epilepsy | Hepatic and hematological abnormalities |
| Increased metabolism of anesthetic agents | |
| Arteriovenous malformation | Potential congestive heart failure |
| Neuromuscular disease | Malignant hyperthermia |
| Respiratory failure | |
| Sudden cardiac death | |
| Chiari malformation | Apnea |
| Aspiration pneumonitis | |
| Hypothalamic/pituitary lesions |
|
Preoperative sedatives given prior to induction of anesthesia can ease the transition from the preoperative holding area to the operating room . Midazolam given orally is particularly effective in relieving anxiety and producing amnesia. If an indwelling intravenous (i.v.) catheter is in place, midazolam can be slowly administered to achieve sedation. Alternatively, sedatives such as barbiturates can be given rectally to induce sleep in preschool children who are uncooperative, and this avoids the use of intramuscular injections. However, methohexital administered rectally has been shown to induce seizures in patients with epilepsy .
Preoperative evaluation and preparation
Closed-claim studies have revealed that neonates and infants are at higher risk for morbidity and mortality than any other age group . Respiratory and cardiac-related events account for a majority of these complications. However, a major pitfall in the management of infants and children for neurosurgery is the presence of coexisting diseases. Given the urgent nature of most pediatric neurosurgical procedures, a thorough preoperative evaluation may be difficult. However, a complete airway examination is essential, because some craniofacial anomalies may require specialized techniques to secure the airway . Most cardiac morbidity due to congenital heart disease occurs during the first year of life . Congenital heart disease may not be apparent immediately after birth, and the hemodynamic alterations caused by anesthetic agents, mechanical ventilation, and blood loss during surgery can unmask these cardiac defects. Echocardiography can be helpful in the assessment of the heart, and a pediatric cardiologist should evaluate patients with suspected problems to help optimize cardiac function prior to surgery. Other coexisting diseases that can alter the conduct of anesthesia are list in Table 1 .
| Condition | Anesthetic implications |
|---|---|
| Congenital heart disease | Hypoxia and cardiovascular collapse |
| Prematurity | Postoperative apnea |
| Upper respiratory tract infection | Laryngospasm and postoperative hypoxia/pneumonia |
| Craniofacial abnormality | Difficulty with airway management |
| Denervation injuries | Hyperkalemia after succinycholine |
| Resistance to nondepolarizing muscle relaxants | |
| Chronic anticonvulsant therapy for epilepsy | Hepatic and hematological abnormalities |
| Increased metabolism of anesthetic agents | |
| Arteriovenous malformation | Potential congestive heart failure |
| Neuromuscular disease | Malignant hyperthermia |
| Respiratory failure | |
| Sudden cardiac death | |
| Chiari malformation | Apnea |
| Aspiration pneumonitis | |
| Hypothalamic/pituitary lesions |
|
Preoperative sedatives given prior to induction of anesthesia can ease the transition from the preoperative holding area to the operating room . Midazolam given orally is particularly effective in relieving anxiety and producing amnesia. If an indwelling intravenous (i.v.) catheter is in place, midazolam can be slowly administered to achieve sedation. Alternatively, sedatives such as barbiturates can be given rectally to induce sleep in preschool children who are uncooperative, and this avoids the use of intramuscular injections. However, methohexital administered rectally has been shown to induce seizures in patients with epilepsy .
Intraoperative management
Induction of anesthesia
The patient’s neurological status and coexisting abnormalities will dictate the appropriate technique and drugs for induction of anesthesia. General anesthesia can be established by inhalation of sevoflurane and nitrous oxide with oxygen. A nondepolarizing muscle relaxant such as pancuronium is then administered to facilitate intubation of the trachea. Alternatively, if the patient has i.v. access, anesthesia can be rapidly induced with sedative/hypnotic drugs such thiopental (5–8 mg/kg) or propofol (3–5 mg/kg). Patients at risk for aspiration pneumonitis should have a rapid-sequence induction of anesthesia performed with thiopental or propofol, immediately followed by a rapid-acting muscle relaxant such as succinylcholine or rocuronium.
Airway management
Developmental differences in the cricothyroid and tracheobronchial tree have a significant impact on management of the pediatric airway. The infant’s larynx is funnel shaped, and narrowest at the level of the cricoid, making this the smallest cross-sectional area in the infant airway. This feature places the infant at risk for subglottic obstruction secondary to mucosal swelling after prolonged endotracheal intubation with a tight-fitting endotracheal tube. Because the trachea is relatively short, an endotracheal tube can migrate into a mainstem bronchus if the infant’s head is flexed, as is the case for a suboccipital approach to the posterior fossa or the cervical spine. Therefore, the anesthesiologist should auscultate both lung fields to rule out inadvertent intubation of a mainstem bronchus after positioning the patient. Nasotracheal tubes are best suited for situations when the patient will be prone and when postoperative mechanical ventilation is anticipated. Furthermore, the endotracheal tube can kink at the base of the tongue when the head is flexed and also lead to pressure necrosis of the oral mucosa.
Maintenance of anesthesia
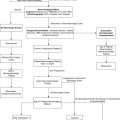
The choice of anesthetic agents for maintenance of anesthesia has been shown not to affect the outcome of neurosurgical procedures . The most frequently utilized technique for neurosurgery consists of the opioid fentanyl administered at a rate of 2–5 μg/kg/h intravenously along with inhaled nitrous oxide (70%) and low-dose isoflurane (0.2–0.5%). Deep neuromuscular blockade is maintained during most neurosurgical procedures to avoid patient movement. Patients on chronic anticonvulsant therapy will require larger doses of muscle relaxants and narcotics because of induced enzymatic metabolism of these agents ( Fig. 1 ) . Muscle relaxation should be withheld, or should not be maintained, when assessment of motor function during seizure and spinal cord surgery is planned.
Fluid restriction and diuretic therapy may lead to hemodynamic instability and even cardiovascular collapse if sudden blood loss occurs during surgery. Therefore, normovolemia should be maintained through the procedure. Normal saline is commonly used as the maintenance fluid during neurosurgery because it is mildly hyperosmolar (308 mOsm/kg), and it theoretically attenuates brain edema. However, rapid infusion of normal saline (30 mL/kg/h) is associated with hyperchloremic acidosis . Hyperventilation and maximization of venous drainage of the brain by elevating the head can minimize brain swelling. Should these maneuvers fail, mannitol can be given at a dose of 0.25 to 1.0 g/kg intravenously. This will transiently alter cerebral hemodynamics and raise serum osmolality by 10–20 mOsm/kg . However, repeated dosing can lead to extreme hyperosmolality, renal failure, and further brain edema. Furosemide is a useful adjunct to mannitol in decreasing acute cerebral edema and has been shown in vitro to prevent rebound swelling due to mannitol . All diuretics will interfere with the ability to utilize urine output as a guide to intravascular volume status.
Vascular access
Due to limited access to the child during neurosurgical procedures, optimal intravenous access is mandatory prior to the start of surgery. Typically, two large-bore venous cannulae are sufficient for most craniotomies. Should initial attempts fail, central vein cannulation may be necessary. Cannulation of a femoral vein avoids the risk of pneumothorax associated with subclavian catheters and does not interfere with cerebral venous return.
Monitoring
Given the potential for sudden hemodynamic instability due to venous air emboli (VAE), hemorrhage, herniation syndromes, and manipulation of cranial nerves, the placement of an intra-arterial cannula for continuous blood pressure monitoring is mandatory for most neurosurgical procedures. An arterial catheter will also provide access for sampling serial blood gases, electrolytes, and hematocrit. The issue of central venous catheterization is controversial. Large-bore catheters are too large for infants and most children, and central venous pressures may not accurately reflect vascular volume, especially in a child in the prone position. Therefore, the risks may outweigh the benefits of a central venous catheter.
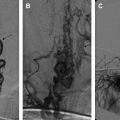
Standard neurosurgical technique may elevate the head of the table to improve venous drainage and is conducive to air entrainment into the venous system through open venous channels in bone and sinuses ( Fig. 2 ) . Patients with cardiac defects, such as patent foramen ovale or ductus arteriosus, are at risk for arterial air emboli through these defects, and should be monitored carefully. A precordial Doppler ultrasound can detect minute VAE and should be routinely used in conjunction with an end-tidal carbon dioxide analyzer and arterial catheter in all craniotomies to detect VAE. A Doppler probe is best positioned on the anterior chest usually just to the right of the sternum at the fourth intercostal space. An alternate site on the posterior thorax can be used in infants weighing approximately 6 kg or less .

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree