, Yves Etienne2 and Maurice Niddam3
(1)
Faculté de Médecine, Marseille, France
(2)
Unité de Médecine Légale, Hôpital de la Timone, Marseille, France
(3)
Unité SAMU 13, Centre 15, Hôpital de la Timone, Marseille, France
The topographic location of this complex and its physiological significance explain that it must be permanently connected to a large number of other cortical and subcortical encephalic structures. The study of the relations of this formation is extremely complex and requires knowledge of both the brain and the brainstem1.
We shall thus study successively the anterior relations, the lateral relations, the superior relations, the distant supero-medial relations, the medial relations and the posterior relations. A paragraph will be dedicated to relations with the brainstem which we shall refer to as the postero-inferior relations.
7.1 Anterior Relations
Laterally and at the temporal floor, the amygdala is connected to the temporal pole. It is connected to the latter by a cylindrical looking bundle, constant during dissections, the fasciculus amygdalo-temporalis of Klinger (see medial relations).
Medially and at the junction between the temporal and frontal levels, the amygdala is surrounded by its periamygdaloid cortex (ambiens and semilunar gyri) and is directly connected to the entire olfactory system,2 especially the prepiriform cortex and the anterior perforated substance, apSb (Fig. 7.1) It is to be recalled that the latter is situated behind the lateral and medial olfactory striae and the olfactory tubercle and that it is limited at the rear, by the optic tract, inside, by the diagonal band and the optic chiasma, and outside, by the endorhinal sulcus, which separates it from the uncus, and by the threshold to the insula or limen insulae, a deep sulcus forming “the bottom of the basal part of the lateral sulcus of Sylvius” (HM Duvernoy 1992). This perforated substance is comprised of paleocortex,3 while the periamygdaloid cortex is made of periarchicortex. In humans, a third stria (referred to as the intermediate stria) is exceptionally linked to the two lateral and medial olfactory striae.4 The triangular space between the lateral and medial striae is the olfactory trigone. Under the base of the trigone, the protrusion of the olfactory tubercle can be observed.
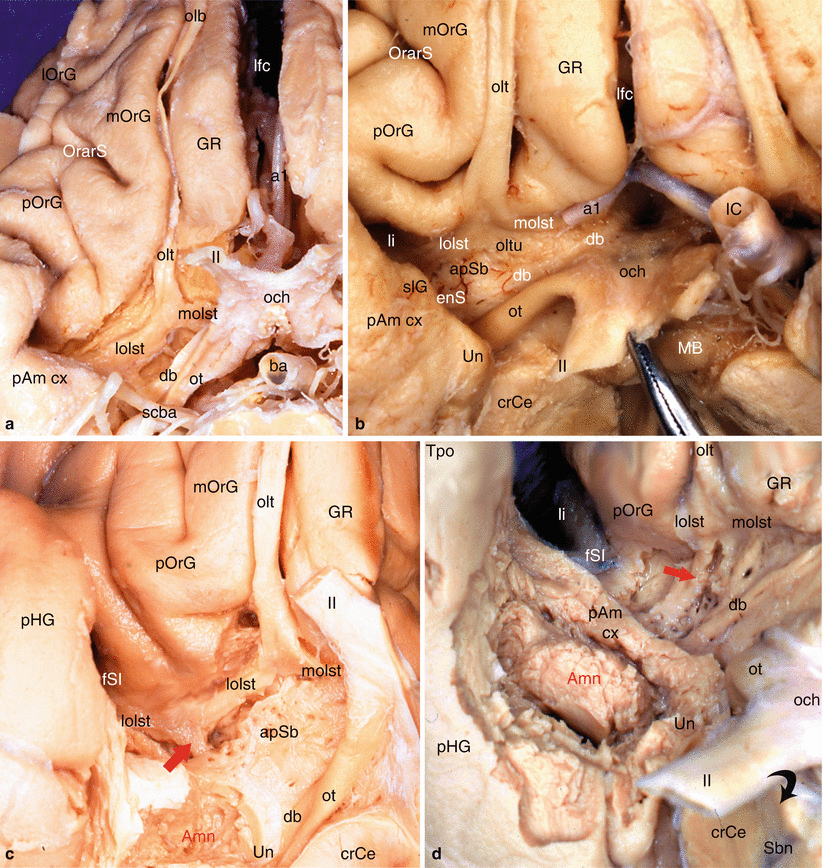

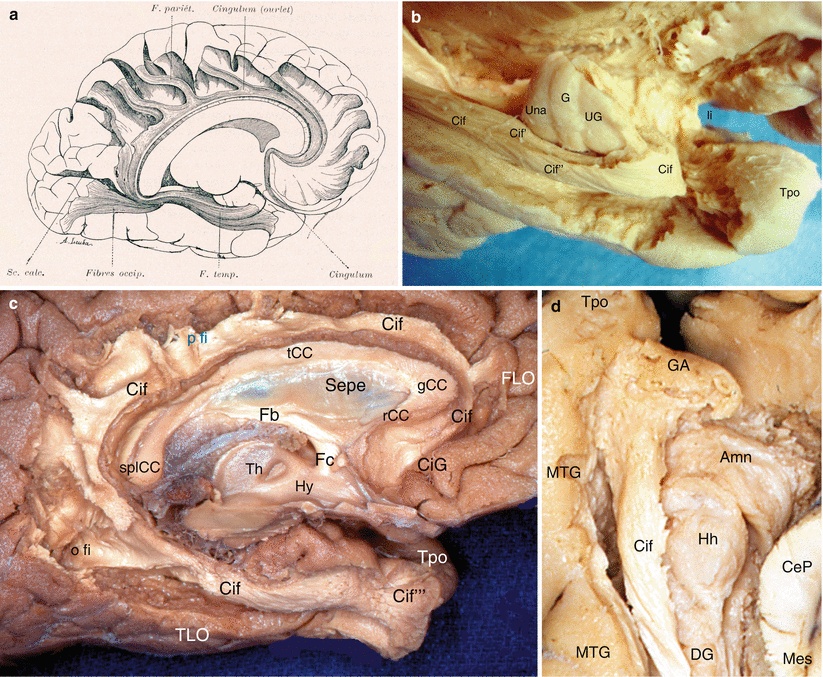
Fig. 7.1
Olfactory system and olfactory pathway to amygdala. (a) Dissection of the right olfactory system on an inferior view of the brain; (b) the right apSb on an inferior view of the brain. (c) Highlighting of the olfactory pathway (red arrow) to amygdala. (d) Highlighting of the olfactory pathway (red arrow) with dissection of the amygdala. Amn amygdaloid nuclear complex, apSb anterior perforated substance, ba basilary artery, crCe crus cerebri, db diagonal band, enS endorhinal sulcus, fSI falciform sulcus of the insula, GR gyrus rectus, IC internal carotid, lfc longitudinal fissure of the cerebrum, li limen insulae, lolst lateral olfactory stria, lOrG lateral orbital gyrus, MB mamillary body, molst medial olfactory stria, mOrG medial orbital gyrus, och optic chiasm, olb olfactory bulb, olt olfactory tract, oltu olfactory tubercle, OrarS orbital arcuatus sulcus, ot optic tract, pAm cx peri-amygalar cortex, pHG parahippocampal gyrus, pOrG posterior orbital gyrus, Sbn substantia nigra, scba superior cerebellar artery, slG semilunar gyrus, Tpo temporal pole, Un uncus, a1 anterior cerebral artery, II optic nerve, red arrows olfactory pathway from lateral olfactory stria to amygdala, curved black arrow it shows the right optic nerve pushed back as on the picture (b)
The tubercle5 receives fibres of the lateral stria. The lateral olfactory stria becomes lost in the limen after having formed, just before the falciform fold (deep notch connecting the planum temporale to the orbital cortex and to the rostral part of the insula), a posterior thin bundle, which is difficult to dissect and which directly reaches the cortical nucleus of the amygdala (see Fig. 7.1). The medial olfactory stria is directed to the median region of the brain and reaches the paraterminal and subcallosal gyri (septal area 25) on the medial surface of the homolateral hemisphere, where is located, in particular, in the anterior olfactory nucleus. The anterior perforated substance (former grey substance of Soemmering6) is “perforated” by multiple small vascular orifices (the largest are lateral) via which penetrate the striated arteries dedicated to the striatum. These rami, which developed from the anterior and middle cerebral arteries at their origin, are therefore vascular connections which are very close to the amygdala (see Fig. 9.1a).
From a topographic viewpoint, the apSb, which is only separated from the amygdala by the endorhinal sulcus and which is only separated from the orbitofrontal cortex by the narrow prepiriformis gyrus, therefore provides a connection between this amygdala and the orbitofrontal cortex (gyrus rectus inside the olfactory tract and orbital gyri outside this tract). Similarly, as it is connected to the limen, the apSb is directly connected to the planum temporale and the insular gyri, and therefore, these formations are connected, via this structure, to the amygdala!
Olfactory inputs (conscious projections), analysed in the olfactory bulb but also in the cortices bordering the posterior third of the olfactory sulcus (medial orbital sulcus), reach, via the lateral olfactory stria and the anterior perforated substance, the primary olfactory centres7 (prepiriform cortex8 and periamygdaloid cortex), the rostral part of the cortical nucleus of the amygdala and, in fine, the secondary projection area, the entorhinal area, area 28, underlying the gyrus ambiens (limbic area where the discrimination between pleasant and unpleasant smells takes place). The inputs also pass via the lateral olfactory stria and reach the limen, via which they reach the insular gyri. This most certainly explains the known links between taste and the smell of food, which interfere in the appreciation of dishes. It is necessary to recall the projections of the primary olfactory centres towards the medial, posteromedial and intermediate agranular insular areas (R Nieuwenhuys et al. 2008).
Furthermore, projection fibres depart from the medial olfactory stria and pass via the medial forebrain bundle (MFB) to reach the hypothalamus and the mesencephalon. Other fibres pass via the stria medullaris thalami to the habenula from which the habenulo-interpeduncular bundle allows the olfactory projection towards the interpeduncular nucleus and the reticular mesencephalic formation.
A major connection of the amygdala in this olfactory area is that of Broca’s diagonal band, which plays several roles within the amygdaloid environment:
It represents the actual postero-internal limit of the apSb (more than the optic tract and the optic chiasm, which are more posterior and do not adhere to the apSb), it has connections to the amygdala itself and to the uncus, and it forms an incredible connection pathway to the septum and amygdala; thus it completes the amygdalo-septal connection implemented via the stria terminalis, by a septo-amygdaloid connection; and therefore forms an actual loop involving the development of amygdalofugal and amygdalopetal fibres.
Our dissections allowed to correctly locate the standard septo-amygdalo-uncal portion, mentioned by all the anatomists since Broca. However, we discovered and highlighted (Figs. 7.2, 7.3, 7.4, and 7.5) a posterior portion of this band, a narrower portion, which was perfectly dissectable and which followed the optic tract up to the pulvinar that it reaches a few millimetres above the top edge of the lateral geniculate body. Therefore, we can assert that the diagonal band is a complex, convex, outward and flattened structure, especially in its initial segment where it is stuck to the apSb, and comprises several parts, a short vertical septal part; an oblique transverse part at the rear and outward (often wrongly called the horizontal part), going from the septum to the unco-amygdaloid junction; and a narrower but more ribboned part, with a slight medial concavity, molded against the lateral surface of the mesencephalon, from the uncus to the thalamus, oblique at the rear and inward. Very rich cellular nuclei occupy three essential locations of the band: a rostral nucleus which is no other than the nucleus known as the “nucleus of the diagonal band”, juxta-septal, a nucleus situated near the amygdala (Fig. 7.5) and the uncus, where the band changes direction (or, if we prefer, immediately before or at the junction of the two segments of the band, at the top of the curvature) and a terminal juxta-thalamic nucleus, at its posterior end.
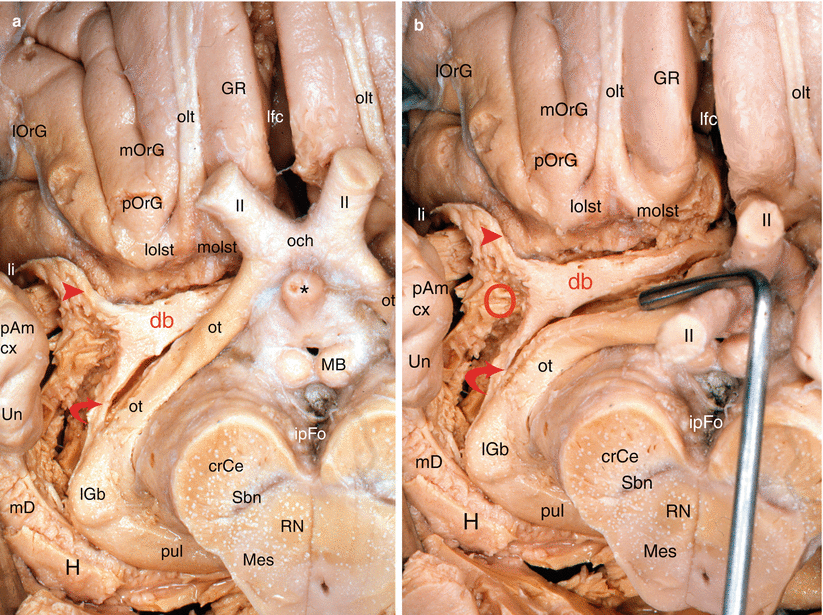
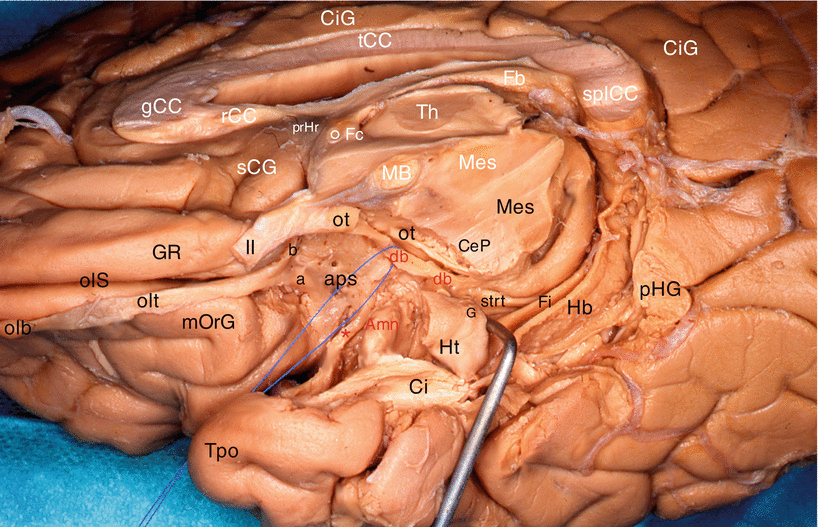
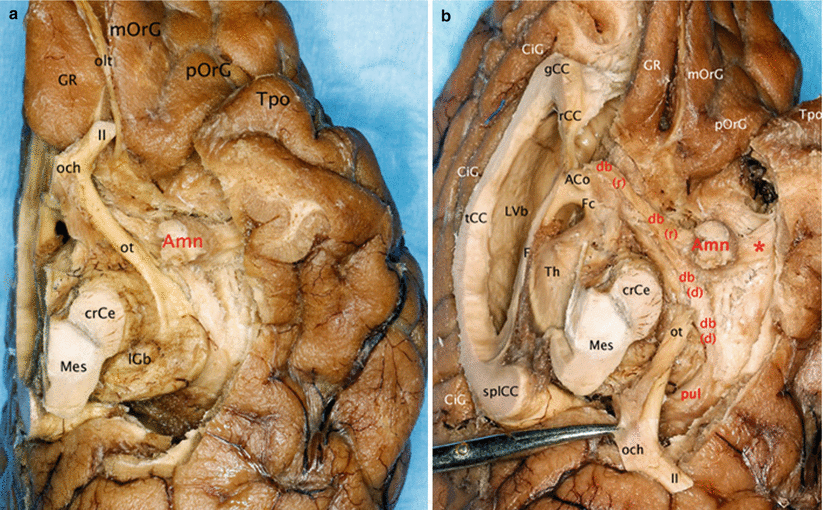
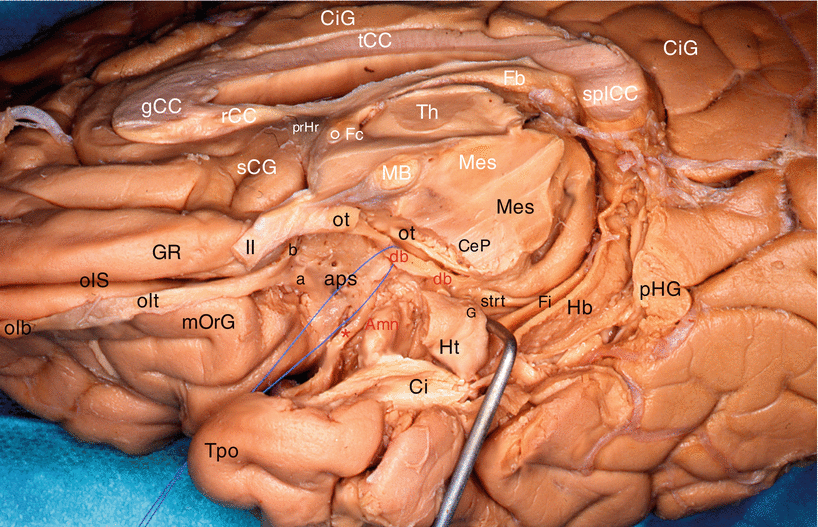

Fig. 7.2
Dissection of the right diagonal band on an inferior view of the brain (the temporal lobe has been tracted laterally. The red circle points the amygdalar area). (a) The optic chiasm is in anatomic position. (b) The optic chiasm has been retracted behind. crCe crus cerebri, db diagonal band, GR gyrus rectus, H hippocampus, ipFo interpedoncular fossa, lfc longitudinal fissure of the cerebrum, lGb lateral geniculate body, li limen insulae, lOrG lateral orbital gyrus, lolst lateral olfactory stria, MB mamillary body, mD margo denticulatus, Mes mesencephalon, mOrG medial orbital gyrus, molst medial olfactory stria, och optic chiasm, olt olfactory tract, ot optic tract, pAm cx periamygdalar cortex, pOrG posterior orbital gyrus, pul pulvinar, RN red nucleus, Sbn substantia nigra, Un uncus. II optic nerve, red arrowhead db pathway to the limen, curved red arrow db pathway to pulvinar, * the asterisk points the infundibular tip of V3

Fig. 7.3
Dissection of the diagonal band on a right cerebral hemisphere. (Infero-medial view of the brain). The fimbria is slightly pushed downwardly by a retractor. For an easy reading, the inscriptions of the sagittal section are white and those of the inferior area are black or red. Amn amygdala, aps anterior perforated substance, CeP cerebral peduncle, Ci cingulum, CiG cingulate gyrus, db (red) diagonal band (identified by a pull wire), Fb fornix (body), Fi fimbria, Fc fornix (column), G band of Giacomini, gCC genu of the corpus callosum, GR gyrus rectus, Hb hippocampus body, Ht hippocampus tail, MB mamillary body, Mes mesencephalon, mOrG medial orbital gyrus, olb olfactory bulb, olS olfactory sulcus, olt olfactory tract, ot optic tract, pHG para-hippocampal gyrus, prHr pre-hippocampal rudiment, rCC rostrum of the corpus callosum, sCG subcallosum gyrus, splCC splenium of the corpus callosum, strt stria terminalis, tCC truncus of the corpus callosum, Th thalamus, Tpo temporal pole, II optic nerve, a lateral olfactory stria, b medial olfactory stria, ° anterior commissure, * peri-amygdaloïd cortex

Fig. 7.4
Relationships of the amygdala with the diagonal band. (a) Inferior view of the left brain hemisphere. (b) Infero-medial view of the left brain hemisphere. (The left optical ways were pulled behind to clear the visibility of the diagonal band.) ACo anterior commissure, Amn amygdaloid nuclear complex, CiG cingulate gyrus, crCe crus cerebri, db diagonal band, db r rostral part, db d dorsal part, F fornix, Fc column of the fornix, gCC genu of the corpus callosum, GR gyrus rectus, lGb lateral geniculate body, LVb lateral ventricle body, Mes mesencephalon, mOrG medial orbital gyrus, och optic chiasm, olt olfactory tract, ot optic tract, pOrG posterior orbital gyrus, pul pulvinar, rCC rostrum of the corpus callosum, splCC splenium of the corpus callosum, tCC truncus of the corpus callosum, Th thalamus, Tpo temporal pole, II optic nerve, * red asterisk it shows the pathway connecting the amygdala and the superior temporal gyrus

Fig. 7.5
Microscopic aspects of the diagonal band. (a) Dissection of the diagonal band (db) showing its constituent parts and its ending in the pulvinar; (b) macroscopic aspect of the db (classical staining); (c) same aspect as b with Luxol fast blue–PAS staining; (d) magnification of the curve between the rostral and dorsal parts of the diagonal band; (e–h) Luxol fast blue–PAS stainings (×200, ×400, ×400, ×200), at the levels a, b, c and d of the diagonal band, on picture a; (i) Luxol fast blue–PAS staining (×25) showing the ending part of the diagonal band in the pulvinar. crCe crus cerebri, db diagonal band, (r) rostral part, (d) dorsal part, fl fasciculus liminis, Mes mesencephalon, och optic chiasm, pul pulvinar, white arrows they show the clusters of neurons at the ending part of db and into the pulvinar. Note the balloon–shaped aspect and the off–centred nuclei of the diagonal band’s neurons
Other observation: our dissections also revealed a double origin of the rostral portion of the band, which is formed not only by the standard septal bundle but also by a slightly arched and relatively thin bundle, coming from the area of the limen9 (Figs. 7.3 and 7.4). Both bundles join before the band reaches the uncus and the amygdala.
Broca’s band is known, at present, to transmit the olfactory inputs to the uncus and septal messages to the amygdala. With the new description defined thanks to our research work, we had to question the meaning of such a band. Sadly, we could not provide a satisfactory answer (oniric pathway? additional junction with the posterior part of the thalamus? Other?). Histochemical colourations and other anatomical dissections and research performed in neurosciences shall undoubtedly and shortly provide essential clarifications. It is to be recalled that Broca’s band is very rich in cholinergic cells and that it is part of the MBF (magnocellular basal forebrain) such as Meynert’s basal nucleus (see Chap. 11). It therefore belongs to a system which sends projections not only to the amygdala and hippocampus but above all to the cortex. Many authors have established correlations between certain cases of senile dementia (including Alzheimer’s disease) and the rarefaction of the cholinergic cells of the MBF. Other authors have referred, in relation to these pathologies, a degeneration of the axons of these cells in their terminal presynaptic part, which is responsible for the loss of the cortical cholinergic markers and the formation of senile plaques (JC Hedreen et al. 1984) and, in fine, the dementia.
7.2 Antero-Superior Relations (Relations with the Frontal Lobe)
The amygdala has, as we have observed, proximity relations with the temporal cortex, that of the apSB and the rostral poles of the cingulate cortex. However, other more distant relations form with a key part of the brain, the frontal lobe, and especially the basal part of the latter.
The cortex of this lobe comprises a dorsolateral surface, a medial surface and a basal or inferior surface based on an orbital cavity.
The dorsolateral surface (Fig. 7.6) on which, from the rear to the front, the precentral motor cortex (area 4 of Brodmann),10 the premotor cortex (area 6)11 and the prefrontal cortex (areas 8, 9, 10, 45 and 46)12 are observed. The prefrontal cortex alone represents a third of the cortical mass!
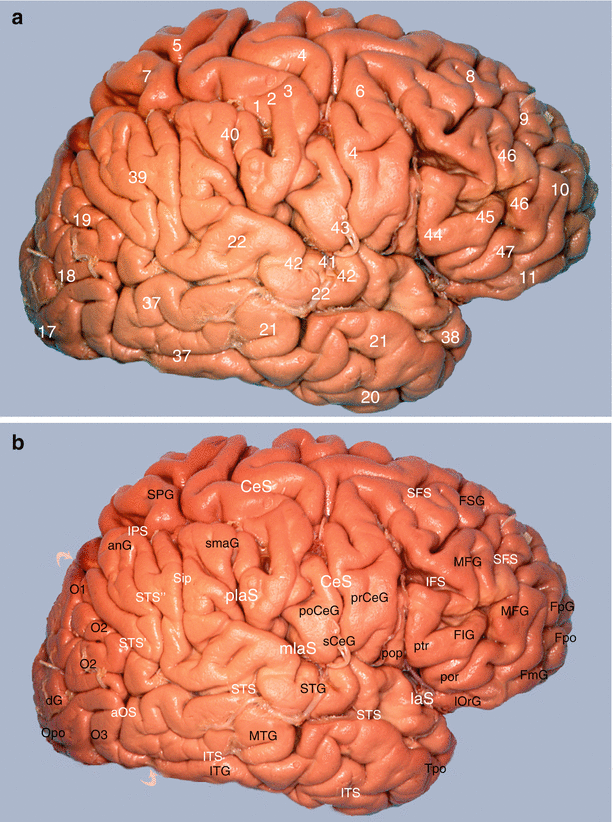

Fig. 7.6
Study of the amygdalo-cortical interrelations. Mapping of the cortical areas according to Brodmann (lateral views of the brain): (a) numbering of areas and (b) correspondences with gyri and sulci. anG angular gyrus, aOS anterior occipital sulcus, CeS central sulcus, dG descendens gyrus, FIG frontal inferior gyrus, pop pars opercularis, ptr pars transversalis, por pars orbitalis, FmG fronto-marginal gyrus, FpG fronto-polaris gyrus, Fpo frontal pole, FSG frontal superior gyrus, IFS inferior frontal sulcus, IPS intraparietal sulcus, ITG inferior temporal gyrus, ITS inferior temporal sulcus, laS lateral cerebral sulcus, mlaS medial lateral sulcus, plaS posterior lateral sulcus, lOrG lateral orbital gyrus, MFG medial frontal gyrus, MTG middle temporal gyrus, O1 superior occipital gyrus, O2 middle occipital gyrus, O3 inferior occipital gyrus, Opo occipital pole, poCeG post-central gyrus, prCeG precentral gyrus, sCeG subcentral gyrus, SFS superior frontal sulcus, Sip sulcus intermedius primus of Jensen, smaG supra-marginalis gyrus, SPG superior parietal gyrus, STG superior temporal gyrus, STS superior temporal sulcus, STS’ posterior part, horizontal segment, STS” posterior part, ascending segment, Tpo temporal pole, curved superior arrow parieto-occipital sulcus, curved inferior arrow temporo-occipital incisure
The medial or internal surface (Fig. 7.7) on which, on the one hand, the medial part of areas 6, 8, 9 and 10 and, on the other hand, rostral limbic areas 24, 25 and 32 are located.
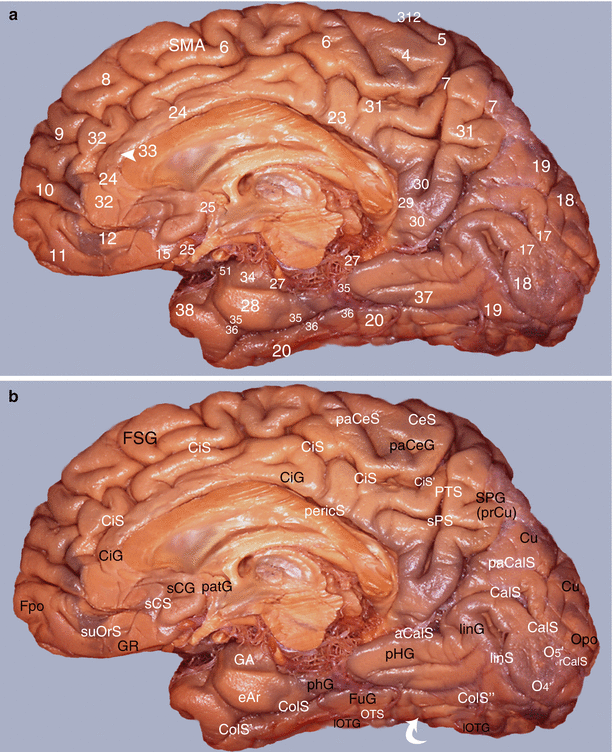

Fig. 7.7
Study of the amygdalo-cortical interrelations. Mapping of the cortical areas according to Brodmann (medial views of the right hemisphere): (a) numbering of the areas and (b) correspondences with gyri and sulci. aCalS antecalcarinus sulcus, CalS calcarine sulcus, CeS central sulcus, CiG cingulate gyrus, CiS cingulate sulcus, CiS’ marginal part of CiS, ColS collateral sulcus, ColS’ transverse anterior collateral sulcus, ColS” transverse posterior collateral sulcus, eAr entorhinal area, Fpo frontal pole, FSG frontal superior gyrus, FuG fusiform gyrus, GA gyrus ambiens, GR gyrus rectus, linG lingual gyrus, linS lingual sulcus, lOTG lateral occipitotemporal gyrus, O4’ posterior part of FuG, O5’ posterior part of linG, Opo occipital pole, OTS occipitotemporal sulcus, paCalS paracalcarine sulcus, paCeS paracentral sulcus, paCeG paracentral gyrus, patG paraterminal gyrus, pericS pericallosal sulcus, pHG parahippocampal gyrus, PTS parieto-temporal sulcus, rCalS retro-calcarine sulcus, sCG subcallosum gyrus, sCS subcallosum sulcus, SMA supplementary motor area, SPG superior parietal gyrus (prCu precuneus), SPS superior parietal sulcus, suOrS sus orbital sulcus, white curved arrow temporo-occipital incisures
The orbital surface, referred to as the orbital cortex (Fig. 7.8), which includes the inferior part of the frontal pole, the medial frontal gyrus, the orbitofrontal gyrus and the inferior part of the cingulate gyrus (areas 10, 11, 12, 25, 15 and 32).
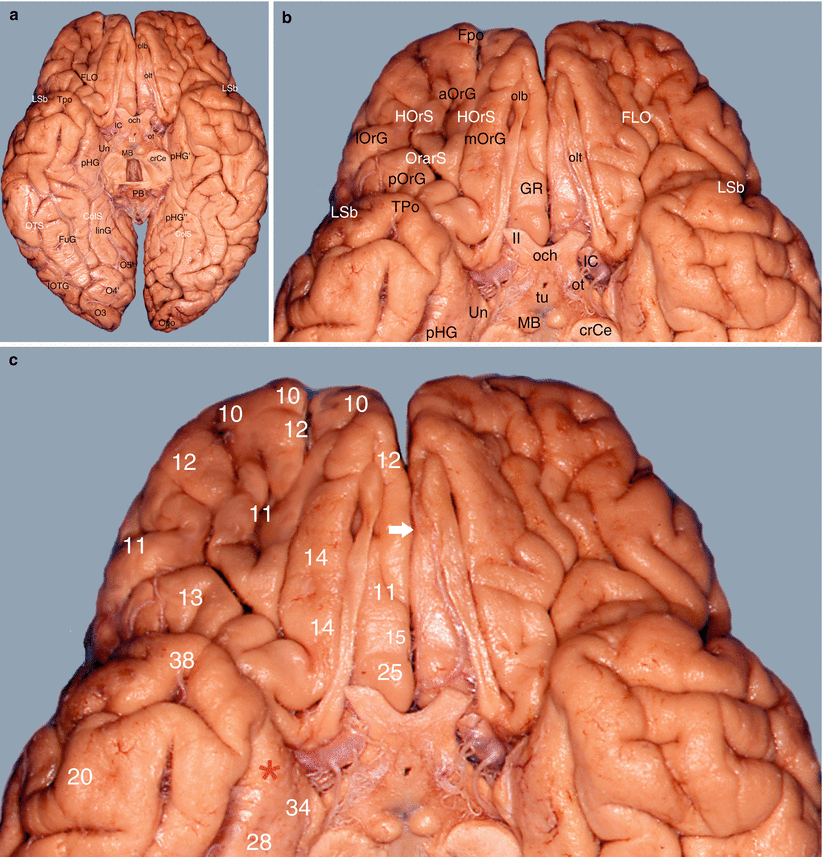

Fig. 7.8
Study of the amygdalo-cortical interrelations. Mapping of the cortical areas according to Brodmann (inferior views of the brain). (a) Basal aspect of the brain, (b) details of the frontal gyri and sulci, (c) numbering of the frontal areas. aOrG anterior orbital gyrus, crCe crus cerebri, ColS collateral sulcus, FLO frontal lobe, Fpo frontal pole, FuG fusiform gyrus, GR gyrus rectus, HOrS H orbital sulcus, IC internal carotid, linG lingual gyrus, lOrG lateral orbital gyrus, lOTG lateral occipitotemporal gyrus, LSb lateral sulcus (basal part), MB mamillary body, mOrG medial orbital gyrus, O3 inferior occipital gyrus, O4’ posterior part of the fusiform gyrus, O5’ posterior part of the lingual gyrus, och optic chiasm, olb olfactory bulb, olt olfactory tract, Opo occipital pole, OrarS orbital arcuatus sulcus, ot optic tract, PB pineal body, pHG parahippocampal gyrus, pHG’ anterior part of pHG, pHG” posterior part of pHG, pOrG posterior orbital gyrus, Tpo temporal pole, tu tuber, Un uncus, II optic nerve, white arrow it shows the longitudinal fissure of the cerebrum (lfC), red asterisk it shows the amygdalar area and its proximity with the basal part of the frontal lobe
Although the dorsolateral surface only has few connections to the amygdala as it is specifically involved in the motor and executive programmes, the orbital and medial surfaces of the prefrontal cortex are very closely connected to the amygdala and all structures governing the brain of emotions.12 Thus, the prefrontal cortex receives direct projections not only from nearby cortices (motor cortex, cingulate cortex, sensorial cortex) but also from associative cortices (temporal, occipital, parietal) from the amygdala, hypothalamus and thalamus: it is therefore permanently informed of the individual’s emotional, somatic, visceral and motivational conditions.
Such as we will see further in the document, the lateral part (lateral frontal gyrus) of the orbital cortex is dedicated to cognition, whereas the medial part (medial frontal gyrus), the orbitofrontal gyrus and the cingulate gyrus are specifically involved in the emotional circuitry. Due to the fact that all of these parts are interconnected and linked to diencephalic structures (thalamus and hypothalamus), basal structures etc., the permanent interrelations existing between cognition, emotions and their corporal expressions, as well as the modulation possibilities of the emotional conditions of the prefrontal cortex, are considered.
7.3 Lateral Relations
The lateral relations of the amygdala are visible on the coronal and axial sections of the brain, but it is, above all, the coronal sections which show the amygdaloid topography the best. The following is very clear on these sections that the amygdala is located in contact with the temporal stem, a narrow junction area between the frontal and temporal lobes. Its lateral surface is adjacent to this temporal stem, which contains not only the grey substance belonging to the claustrum but also all of the fibres connecting these lobes, whether they are ascending or descending, and therefore has a major significance.
Although this stem has already been studied, even recently, by many teams, by using dissection and (or) radiography (J. Peltier et al. 2010, EL Kier et al. 2004, CY Choi et al. 2010), the topic subject to discussion remains the limit of this stem. Where is the limit between the frontal and the temporal lobe to be set? The question concerns both the lateral end of this limit (inferior circular sulcus of the insula, limen, sylvian valley?) as well as the medial end (roof of the inferior horn of the lateral ventricle, tail of the caudate nucleus, endorhinal sulcus?). According to our experience, it appears that concerning the sections providing the best view of this amygdala, this limit corresponds to the shortest line drawn on the fronto-temporal neck from the recess of the endorhinal sulcus.
The relations of the amygdala with the isthmic white substance are multiple:
With the uncinate fasciculus, these connections are particularly remarkable (Figs. 7.9, 7.10 and 7.11). It is to be recalled that this arch-shaped fasciculus, a large fibrillar system which is junctional between the frontal and temporal lobes, comprises three parts: a frontal part, oblique at the top and front, which has developed, on the one hand, in the middle frontal lobe and the frontal pole and, on the other hand, in the orbital gyri and gyrus rectus; an intermediate part, the isthmus or infra-insular segment inside which the fibres combine, is curved and concave in the front region and covers the limen; and a temporal part, which is nearly horizontal and whose fibres have developed in the middle and Zinferior temporal gyri and the temporal pole. In 2004, EL Kier et al. showed, by means of MRI techniques studying previously dissected anatomical portions, that the frontal fibres of the uncinate fasciculus were routed in the external capsule and in the extreme capsule (a few fibres originate from the claustrum), prior to penetrating inside the middle part of the temporal isthmus. The uncinate fasciculus then nears the amygdala with which its intermediate part has developed close connections. The uncinate fibres border the lateral surface of the lateral nucleus of the amygdala and combine with them (Fig. 7.9). Others are distributed inside this lateral nucleus or penetrate it. This provides a comb tooth-type aspect to its most lateral part. A recent microanatomical study performed on the fibres of the uncinate fasciculus (N Travers 2008) refers to similar connections to the baso-lateral nucleus of the amygdala. It must also be noted that certain fibres of this uncinate fasciculus seem to be originated from the periamygdaloid cortex, next to the cortical nucleus.
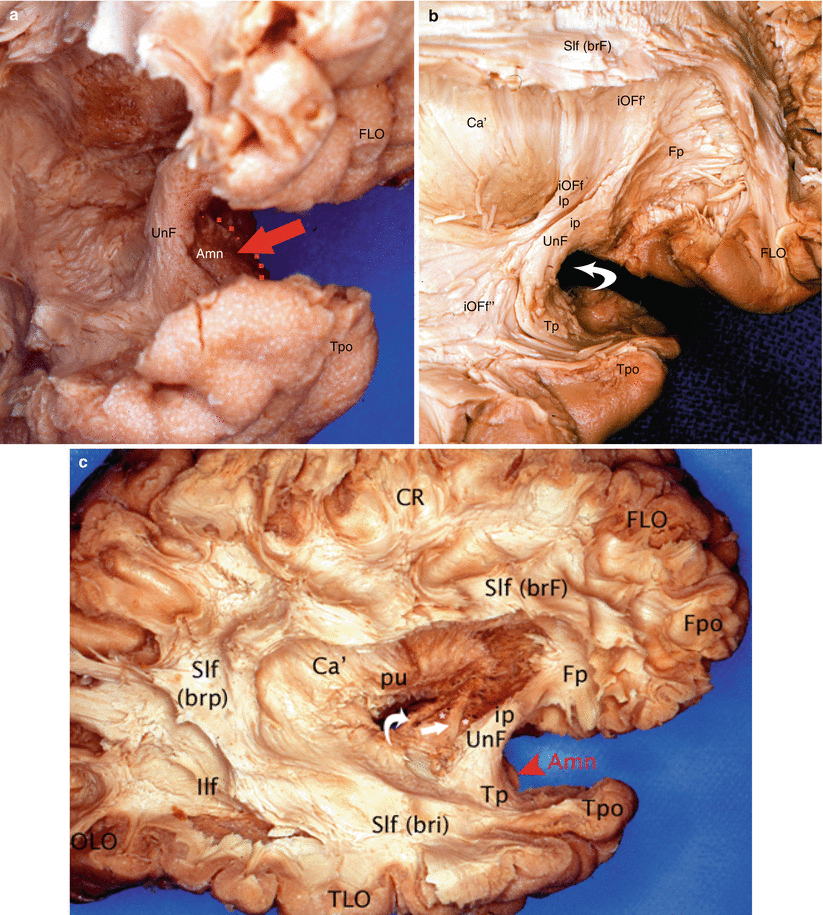
Fig. 7.9
Dissection of the lateral surface of the right hemisphere to illustrate the uncinate fasciculus and its relation to the amygdaloid nuclear complex. (a) 3/4 view in order to have a good visibility of the Amn joined with the medial aspect of the UnF; (b) dissection of the UnF. Note the IOFf adjoining to the isthmus of UnF. (c) Lateral dissection of the right hemisphere to localise Amn, UnF, ACo and ot. Amn amygdaloid nuclear complex (red arrow and red arrowhead), Ca’ external capsule, FLO frontal lobe, Fpo frontal pole, Ilf inferior longitudinal fasciculus, iOFf inferior occipito-frontal fasciculus (Ip insular part, iOFf’ frontal part, iOFf” temporal part), OLO occipital lobe, pu putamen, Slf superior longitudinal fasciculus (brF, brp, bri brachium frontal, posterius and inferius), Tpo temporal pole, UnF uncinate fasciculus (ip, Fp, Tp isthmic frontal and temporal parts), white curved arrow on b it shows the limen, right arrow on c ACo (anterior commissure), asterisks amygdalofugal pathway, white curved arrow on c ot (optic tract)
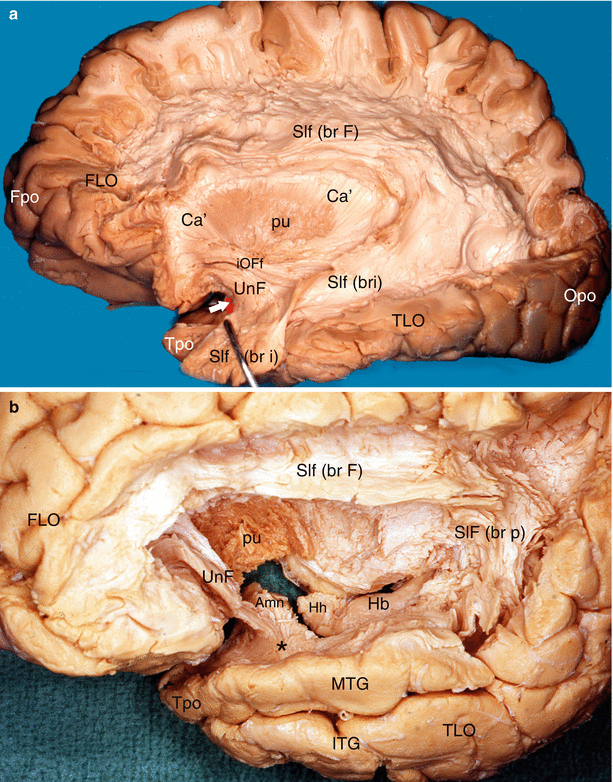
Fig. 7.10
Dissections of the lateral surfaces of two left hemispheres to precise the relations of the Amn with the uncinate fasciculus. (a) The white arrow shows the anterior edge of the amygdala (red coloured). (b) The superior temporal gyrus has been resected in order to show exactly the relations. Amn amygdaloid nuclear complex, Ca’ external capsule, FLO frontal lobe, Fpo frontal pole, Hb hippocampus body, Hh hippocampus head, iOFf inferior occipito-frontal fasciculus, ITG inferior temporal gyrus, MTG middle temporal gyrus, Opo occipital pole, pu putamen, Slf superior longitudinal fasciculus, br F brachium frontalis, br p brachium posterius, br i brachium inferius, TLO temporal lobe, Tpo temporal pole, UnF uncinate fasciculus, black asterisk it shows the fibres of the uncinate fasciculus joining the white matter of the MTG
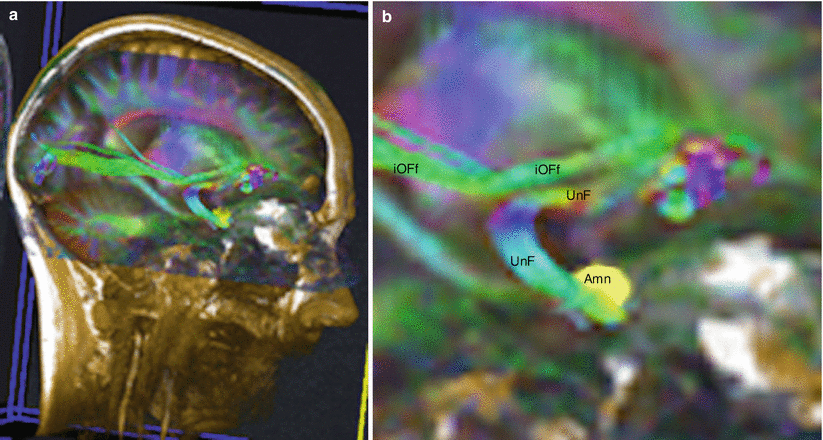
Fig. 7.11
Diffusion tensor imaging fibres tracking (DTI) of the uncinate fasciculus to show its relations with Amn. (a) Location on the head; (b) enlargement of the fasciculi seen on the previous picture a. Amn amygdaloid nuclear complex, UnF uncinate fasciculus, iOFf inferior occipito-frontal fasciculus
With the inferior occipito–frontal fasciculus, the uncino-amygdaloid connections also concern the middle part of this fasciculus. The inferior occipito-frontal fasciculus is, in fact, a long fasciculus presenting a postero-superior concavity, adjacent to the uncinate fasciculus over the part of its route (D Peuskens et al. 2004). It connects the prefrontal cortex of the frontal lobe to the posterior part of the temporal lobe (at the level of the lingual and fusiform gyri) and to the occipital lobe (up to the cuneus). This fasciculus, such as the uncinate bundle, comprises three parts: a frontal part, an occipito-temporal part and an insular intermediate part. The latter is adjacent to the uncinate isthmus, whose dorsal part it is positioned against, which is the reason why it was often believed that the inferior occipito-frontal fasciculus is the superior part of the uncinate fasciculus (J Sedat and H Duvernoy 1990). The persistent controversy concerning the capsule, inside which passes the inferior occipito-frontal fasciculus to each the temporal stem (extreme capsule for E Curran 1909, external and extreme capsules for JM Edeline and NM Weinberger 1992, for EL Kier et al. 2004, for J Peltier et al. 2010), is to be noted. It however remains certain that the inferior occipito-frontal fasciculus shares, with the uncinate fasciculus, contiguity connections to the amygdala and that some of its fibres are probably involved in the partition of the lateral part of the amygdala, which therefore provides the external part of its lateral nucleus with a streaked aspect.
With the Anterior commissure, relations are close as part of the commissure fibres directly connect, as we have already observed (see Chap. 6), the two amygdalae, which they penetrate at the level of their respective baso-lateral nuclei, therefore forming the inter-amygdaloid component, which allows a direct connection between the left and right amygdalae. These amygdaloid fibres originate at the penetration of the lateral expansion inside the temporal stem. Other fibres, belonging to the inter-hippocampal component, originate at the same level and reach the hippocampus. The expansion of the commissure follows its route against the lateral surface of the amygdala, in a medial situation with respect to the uncinate and inferior occipito-frontal fasciculi. In this case, the expansion appears as “the diagonal of a rectangle whose two parallel edges would be the uncinate bundle and the optic tract” (J Peltier et al. 2010). When they reach the inferior edge of the amygdala, the expansions develop and disperse to form temporal components which essentially reach the middle and inferior temporal gyri.
The amygdala does not seem to have any relation with the corpus callosum. However, as remarked by J. Peltier et al. (2010), “A short portion of the radiations of the CC seems to belong to the temporal stem”, which therefore represents a potential relation with the amygdala as the amygdala is adjacent to the stem. Furthermore, the knowledge of these radiations is major: as shown by EC Crosby et al. (1962), the ventral fibres of the corpus callosum pass inside the extreme capsule, penetrate the temporal stem and bypass the cortex of the inferior sulcus of the insula to end in the superior cortex of the superior temporal gyrus. However, for the amygdala, this is only a proximity relation because the fibres of the anterior commissure will be interposed between the amygdala and callosal radiations, underneath which they pass to reach all of the parts of the temporal cortex.
The amygdala has successively developed the following relations with the inferior peduncle of the thalamus:
Distant lateral relations, with its section which is developed on different parts of the temporal cortex (superior temporal, middle temporal and inferior temporal), parts corresponding to the inferior thalamic radiations which forward to the thalamus and the sensitive and sensory inputs from the temporal cortex.
Closer relations with the converging part of these radiations which form a pathway, the inferior thalamic peduncle, which will pass through the temporal stem to reach the thalamus. Along this route, the peduncle receives from the amygdala the ventral amygdalofugal pathway and especially, in its anterior part, the amygdaloid fibres for the thalamus (see Chap 6).
During a lateral dissection of the hemisphere, it is possible to observe this ventral pathway between the optic tract and the anterior commissure, in a medial location with respect to the latter (see picture c, Fig. 7.9).
The amygdala contracts contiguity relations with the temporal loop (“Adolph Meyer’s loop, 1970”, or “détour de Meyer” of French authors) (or geme temporalis of Putman), initial portion of the optic radiations which are freed from the lateral geniculate body. The anterior pathway of the optic tract fibres is briefly routed towards the roof of the inferior horn of the lateral ventricle. It then skirts round the end section of this horn to pass at a ventral level and form Meyer’s loop or temporal knee of the optic radiations. It is this knee which is close to the posterior part of the lateral surface of the amygdala. It is separated therefrom by the most posterior fibres of the anterior commissure, which themselves are applied against the posterior convexity of the uncinate isthmus (U Ebeling and HJ Reulen 1988). Beyond the loop, the optic radiations move further away from the amygdala and pass under the inferior longitudinal fasciculus, in the sagittal stratum, with the most medial fibres of the inferior occipito-frontal fasciculus and a few fibres of the anterior commissure, in the direction of the occipital lobe.
Overall, it can be observed that the lateral fascicular relations of the amygdala explain the communication possibilities of this formation with the frontal and temporal areas. The medial fascicular relations will show similar possibilities with the entire limbic lobe.
The lateral relations with the grey matter concern only the peduncle of the lenticular nucleus and the ventral part of the claustrum. The latter, which belongs to the temporal stem from a topographic viewpoint, is contiguous with the lateral surface of the amygdala (see Fig. 4.9). Such as the lateral amygdaloid nucleus, the claustrum is dissociated by the fibres of the uncinate fasciculus and therefore appears fragmented. The claustrum portions which are the nearest to the amygdala are the most anterior and medial: pre-amygdalar claustrum and peri-amygdalar claustrum. The most lateral portions, which are relatively contiguous with the inferior limiting circular sulcus of insula, are the temporal claustrum, the limitans claustrum and the diffuse insular claustrum.
As for the peduncle of the lenticular nucleus, which sometimes is sus-isthmic but generally sub-isthmic, it can be developed and descend below the isthmic limit and appear as a triangular-shaped appendix of the putamen. It is located laterally and at the rear of the anterior commissure (see picture a, Fig. 4.11).
7.4 Medial Relations
7.4.1 Relations with the Cingulate Fasciculus
The most immediate connection of the amygdala concerns the inferior end of the cingulate fasciculus whose concave surface envelopes it such as “a chistera” in a game of “Basque pelota”.
It is to be recalled that this white substance fasciculus known as the “faisceau du crochet” according to French anatomists, since Foville,13 nearly surrounds the entire medial surface of the cingulated gyrus, from the anterior end, located inside the subcallosal gyrus, under the rostrum of the corpus callosum. The rostral part of the fasciculus bypasses the knee of the corpus callosum, while its posterior part bypasses the splenium of this formation, prior to passing inside the parahippocampal gyrus. It receives, along its entire circumference, white fibres which descend vertically from the cortical areas and then are curved and are incorporated therein. Some fibres ascend again towards one or several cortical areas that are more or less close (Fig. 7.12). Some fibres of the fasciculus are long and connect its antero-superior and inferior ends.