5 Stereotactic Interventions X-ray mammography is currently the standard diagnostic imaging modality for the early detection of breast cancer. It is the most effective method for the detection of calcifying early-stage breast cancer and has a high sensitivity for the detection of suspicious masses in fatty and fibroglandular breast tissue. Nevertheless, the diagnostic sensitivity of x-ray mammography in dense breast parenchyma (American College of Radiology [ACR] density types III and IV) is significantly limited, and noncalcifying tumors (70% of all breast cancers) are often obscured in such mammo-grams. Studies indicate that the mammographic sensitivity for the detection of breast cancer is reduced to approximately 40% in extremely dense breast tissue (ACR density type IV), and to approximately 60% in heterogeneously dense breast tissue (ACR density type III). The specificity of mammography is approximately 80–90%. Screening mammography is the term used for x-ray mammography incorporated in early breast cancer detection programs aimed at women without clinical symptoms with the goal of detecting cancer in its early stages, thus reducing breast cancer mortality. International professional associations vary in their recommendations with regard to screening, but most agree on examination intervals between 1 and 2 years beginning at 40 to 50 years of age. Women presenting with clinical symptoms indicative of breast cancer undergo diagnostic mammography. Follow-up mammography after breast cancer treatment is recommended biannually for the affected breast for the first 3 years after diagnosis, and annually for the contralateral breast. High-risk women may initiate a mammogram screening program at the age of 30 years, or at an age 5 years younger than the youngest affected family member. The low energy x-rays used in mammography produce high-contrast images that differentiate between normal breast tissue and possible abnormalities. Special film-screen combinations and digital detectors promote high spatial resolution. Breast compression while undergoing a mammography is necessary for optimal image quality. Compression spreads tissue out so that small abnormalities are less likely to be obscured, allows a lower x-ray dose to be used to image the breast, and minimizes motion artifacts and x-ray scatter. Standard mammographic projections are the craniocaudal view (CC) and the mediolateral-oblique view (MLO). A strict mediolateral (ML) or lateromedial (LM) view may be additionally performed for optimal spatial orientation. Magnification views and focal/spot compression views are the most common special mammography views used to facilitate evaluation. At present, two techniques are available for the performance of mammography: a conventional film-screen technique and a digital technique. The digital technique offers several advantages. Postprocessing, including window leveling for optimal contrast and brightness, image inversion, and zooming, as well as the application of a computer-aided detection system (CAD), can be used to help interpret digital mammograms. Digital radiographic images can be stored and sent electronically, and the required radiation dose is reduced by 25 to 30% in comparison to conventional film-screen mammography. Digital tomosynthesis mammography and dual-energy contrast-agent—enhanced digital subtraction mammography are digital applications currently under investigation. Quality assurance. All components of mammographic imaging are regularly subjected to quality assurance checks. This is true for factors involved in the performance of an examination, as well as those pertaining to image processing and digital imaging. The quality of mammographic images is evaluated and categorized according to the PGMI (perfect, good, moderate, inadequate) rating system (Table 5.1). The criteria used for evaluation relate to image labeling, film processing, and breast positioning, as well as to standard views. Mammograms performed after breast conservation therapy, reduction mammoplasty, or breast augmentation are not included in the quality evaluation.
X-Ray Mammography
Significance of X-ray Mammography in Breast Diagnostics
Indications
Technique and Methodology
PGMI classification |
P = perfect |
G = good |
M = moderate |
I = inadequate |
Quality assurance standards* |
> 75% of image pairs should be classified as P or G |
> 97% of image pairs should be classified as P, G, or M |
< 3% of image pairs should be classified as I |
* One evaluation is given to an image pair (right and left MLO view, right and left CC view).
Fig. 5.1 Evaluation criteria for mass lesions. Definitions used for shape, margins, and density.
Terminology and Diagnostic Criteria in X-ray Mammography
The ACR BI-RADS Mammography Lexicon is the basis for mammographic image analysis. Findings are described using a standardized language, and categorized according to the probability of malignancy.
Masses and densities. A mass is a space-occupying lesion seen in two different projections. A density is a potential mass seen in only one projection. The assessment of these lesions takes the features shape, margins, and density into account (Fig. 5.1). If the shape of a lesion is oval or round, it is more likely to be benign than an irregularly shaped or spiculated lesion. Circumscribed margins are usually a sign of benignity; indistinct margins could indicate malignancy. The probability of malignancy is lower for low-density lesions than for high-density lesions.
Calcifications. Calcifications can be divided into two groups according to their size.Macrocalcifications are usually benign. Microcalcifications (< 0.5 mm) are sometimes associated with malignancy. A description of calcifications should include their morphology and their distribution (Fig. 5.2). Morphologically they are divided into typically benign calcifications, e. g., round or punctate, intermediate concern calcifications, i. e., amorphous or coarse heterogeneous, and higher probability of malignancy calcifications, i. e., fine-pleomorphic and fine-linear branching.
Distribution modifiers describe the arrangement of the calcifications as grouped or clustered (< 2 cm3), linear, segmental, regional, or diffuse. A cluster of microcalcifications might indicate malignancy. A segmental or linear arrangement, however, is considered more suspicious of malignancy.
To facilitate assessment of microcalcifications, a matrix is currently being employed in clinical studies (Fig. 5.3).
Architectural distortion. An architectural distortion is an alteration of the normal breast without an associated visible mass, and is another criterion used in mammographic assessment. If not associated with prior surgery or trauma, further diagnostic work-up is required.
ACR BI-RADS mammography assessment categories. The purpose of a structured analysis and consistently using standardized language in the description of breast lesions is to help categorize these according to their probability of malignancy, and thus aid in decision making as to what consequences to take. The ACR BI-RADS for mammography has defined seven categories for lesion assessment (Table 5.2).
Fig. 5.2 Microcalcifications. Morphology and distribution patterns.
Fig. 5.3 Matrix to assess suspiciousness of microcalcifications according to Müller-Schimpfle. The x-axis shows increasing suspiciousness of morphology to the right. The y-axis shows increasing suspiciousness of distribution toward the bottom. The corresponding BI-RADS classification is indicated at the crossing.
Basic Principles of Stereology
The design of all stereotactic breast intervention systems is the same. They must provide for breast positioning and compression, acquisition of scout and stereo x-ray-images, determining and transferring target coordinates, and guiding the needle/biopsy instrument to the exact target position.
Lesion depth. The principle for determining the depth of a target lesion is the same for all stereotactic systems. First, a scout image demonstrating the abnormality in the biopsy window is acquired. Then, two images are acquired at +15 ° and –15 ° angles to form the stereo pair (Fig. 5.4).
The resulting shift of a target reference point on these images is proportional to the lesion depth (position in the z-axis): the further away an abnormality is from the image detector, the greater the distance of the abnormality shift is in the stereo pair. Knowing the angle at which the x-ray tube is pivoted for acquiring the stereo pair (+15 ° and –15 °), modern digital systems automatically calculate the depth of an abnormality based on the tangent function of the right triangle shown in Fig. 5.5.
Fig. 5.4a–c Stereotactic table viewed from above (without patient padding). X-ray tube in neutral, 0 ° position (a), and after pivoting 15 ° to the right (b) and left (c).
Fig. 5.5 Lesion depth. The displacement of a lesion in the image plane (x) by pivoting the x-ray tube ± 15 ° correlates to the distance of the lesion from the detector (zd).
Stroke margin. Modern stereotactic biopsy systems also monitor the stroke margin. The stroke margin is the distance between the needle tip and the detector/breast support after firing the biopsy gun. When the stroke margin falls below the tolerance value, firing the biopsy gun runs the risk of injuring breast tissue and skin on the distal breast surface, as well as damaging the unit detector. This can be a painful and expensive undertaking.
Calculation of the stroke margin requires knowledge of the needle tip’s end position after firing. Because this is dependent upon the needle length and stroke—and the stroke of different biopsy needles varies—the unit must be programmed accordingly to ensure accuracy. This can be accomplished in different ways. Most equipment manufacturers provide a computer file containing all available needle lengths for selection at the beginning of an intervention. Alternatively, the needle tip position can be calibrated using a notch and bead sight. Using this method, the target cannot be marked before calibration has been completed.
Calibration. Verification of the accuracy of the stereotactic system’s calibration is performed daily or prior to use by comparing the correct or standard measurements assigned to a point in space with the measurements made by the stereotactic system. Each stereotactic system has its own specific add-on calibration target. A consistency check must be performed once a month.
To test the Lorad table (Fig. 5.6a), a calibration needle is first set to the zero position in the notch and bead sight. Then the x-, y-, and z-coordinates are manually set at 10, 20, and 30 mm. The needle tip is marked in the stereo pair images and the coordinates shown by the stereotactic unit must match these values.
The Fischer table (Fig. 5.6b) uses a phantom with needle tips that are localized stereotactically. Correct calibration is indicated by the exact agreement of the phantom and biopsy needle tip positions.
Specific Patient Information
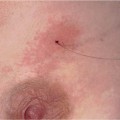
Before beginning the procedure, the patient must be informed in detail about what she may expect. Most are especially surprised to hear that the greatest problem they will probably encounter is having to lay still in the prone position for 30–45 minutes. Explaining the procedure steps gives the patient a feeling of security and helps the patient to maintain the patience required for the procedure. The need for repeated x-ray imaging to check the needle position as well as the necessity of harvesting multiple samples should be discussed. It is also important to reaffirm that no anticoagulant medication such as aspirin or coumarin derivatives have been taken, and to ascertain whether the patient has had prior allergic reactions to local anesthetics, adhesive bandages, and nickel (localization wire, clip). The patient should be informed about possible complications such as bleeding, infection, and allergic reactions. Note that after the intervention, the biopsy area will be cooled and compressed for approx. 30 minutes. If microcalcifications are to be biopsied, the patient should know that the tissue cores will be radiographed to ensure that they contain the calcifications sampled, and that this signifies correct sampling.
It is advisable to demonstrate the noises associated with firing the biopsy gun and harvesting the biopsy cores before beginning the procedure.
Possible problems. Certain situations can create problems for performing stereotactic interventions and should be discussed with the patient when appropriate. These include (1) the thin breast, which is not of sufficient compressed width to accept the length of the needle throw; (2)very fine microcalcifications, which are sometimes difficult to detect on stereo images, and (3) the mass lesion, which is sometimes difficult to target appropriately for correct coordinate calculation. In particular, lesions near the chest wall or skin can make a stereotactic intervention difficult to perform.
Stereotactic Vacuum-assisted Biopsy
Vacuum-assisted biopsy (VAB) is the standard method for performing a stereotactic biopsy. Instead of acquiring tissue samples by a spring-loaded movement of the biopsy needle (large core biopsy), vacuum biopsy instruments suction tissue into a side notch (sampling chamber), and depending on the biopsy system, shear the specimen by slowly advancing the rotating inner or outer cutting cannula (Fig. 5.7). The biopsy specimen is then either transported by vacuum through the cannula to the specimen collection chamber, or removed from the breast with the needle through a coaxial introduction needle. The main advantages of VAB are that a larger tissue volume is removed, and that the sampling notch can be rotated 360 ° so that multiple contiguous cores can be obtained around the centrally located needle. Professional associations recommend obtaining ≥ 12 samples when using an 11-gauge needle.
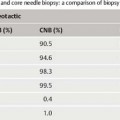
VAB has a higher sensitivity than core biopsy in the stereotactic diagnostic work-up of suspicious microcalcifications. This is due to sampling error encountered when performing a core biopsy of microcalcifications (Fig. 5.8). Because conventional core biopsy obtains noncontiguous cores of tissue, it is possible for the separate cores to miss passing through the tissue containing the target calcifications.
Fig. 5.6a–g Stereotactic equipment. Stereotactic biopsy tables (a–c: patient in prone position): (a) Lorad table, (b) Fischer table, (c) Giotto table. Stereotactic equipment (d: patient in inclined position): (d) Senograph DS (GE Healthcare, Chal-font St., Giles, UK). Stereotactic equipment (e–g: patient in sitting position): (e) Selenia Digital Mammography (Hologic, Bedford, MA), (f) Senograph DS, (g) Siemens Novation (Siemens Medical Solutions, Erlangen, Germany).
Fig. 5.7a, b Sampling method for two different vacuum-assisted devices. After vacuum suction pulls the breast tissue into the sample notch of the biopsy needle, the rotating cutter shears the specimen from the adjacent tissue. The Mammotome (Ethicon Endo-Surgery, Cincinnati, OH) and ATEC systems (Suros Surgical Systems, Inc., Indianapolis, IN) have an inner rotating cutting stylet (a). The Vacora system (Bard Biopsy Systems, Tempe, AZ) has an outer cutting cannula (b).
Fig. 5.8a, b Sampling error. Large CNB of microcalcifications with sampling error (a). Vacuum biopsy obtains representative samples containing microcalcifications by acquiring contiguous specimens around central needle position (b). (Circles = biopsy cores, stars = microcalcifications).
Indications and Goals
The most common indications for the performance of VAB are mammographic findings in the BI-RADS categories 4 and 5 that do not have a correlating ultrasound (US) finding. With the carcinoma risk of these lesions ranging from 2 to 100%, one cannot forgo histologic assessment. Occasionally, findings in the BI-RADS category 3 are also subjected to biopsy (e. g., patient wishes).
The main indication for performing a stereotactic VAB is the histologic assessment of suspicious microcalcifications. The goal of performing stereotactic VAB is to avoid unnecessary surgery when ambiguous imaging findings are found to be benign on histology, and to verify a breast cancer diagnosis for optimal preparation before definitive treatment. In rare cases, VAB can be used to completely excise small borderline lesions, avoiding the need for surgical excision.
European S3 Guidelines for the Performance of Percutaneous Breast Biopsies
Percutaneous breast biopsy is indicated for histologic assessment of imaging findings in the BI-RADS categories 4 and 5. The goal is to verify breast cancer for better planning before definitive therapy is initiated, as well as to rule out cancer and avoid unnecessary open surgery for lesions of benign histology.
ACR Practice Guidelines for the Performance of Percutaneous Breast Biopsies
Indications for percutaneous breast biopsies include, but are not limited to, the following: lesions assessed as highly suggestive of malignancy (BI-RADS Category 5) to confirm the diagnosis so that definitive treatment options can be selected; lesions assessed as suspicious abnormalities (BI-RADS Category 4); multiple suspicious masses, particularly in a multicentric distribution to facilitate treatment planning; and lesions assessed as probably benign (BI-RADS Category 3) when there are valid clinical indications, rebiopsy when initial biopsy results are discordant with the imaging assessment.
Suspicious microcalcifications. Microcalcifications constitute the most common indication for the performance of stereotactic VAB. Usually, there is no correlating finding on US. Taking into account that the average positive predictive value (PPV) is 40% for microcalcifications in the BI-RADS categories 4 and 5, it is indispensable that the indication for performing open surgery be verified by prior percutaneous biopsy. Specimen radiography allows the peri-interventional verification of successful sampling. Confirmation of target calcifications in the biopsy cores substantiates that the sample is representative.
Mass lesions. Aside from microcalcifications, mass lesions detected on mammography are another indication for stereotactic biopsy, provided they are not seen on US. It is recommended that biopsy be performed as a VAB. In justified cases, however, it may be performed as an automated core needle biopsy (CNB). When there is the possibility that the whole mass lesion may be removed by the needle biopsy procedure, a clip marker should be placed at the biopsy site.
The stereotactic determination of a mass lesion’s depth is sometimes very difficult. In contrast to the situation when biopsying microcalcifications, where a calcification can be singled out to serve as a target, choosing a reference point in the +15 ° and −15 ° stereo images of a mass lesion is not possible if no distinguishing mark can be identified.
Architectural distortion. Architectural distortions can also be percutaneously biopsied by means of a stereotactic VAB. If histology reveals a radial scar, open biopsy is warranted because there is a possible association with carcinoma development, usually of the tubular type, in the periphery of such lesions.
Materials
Mammotome biopsy system. The Mammotome biopsy system (Ethicon Endo-Surgery, Cincinnati, OH) was introduced in the late 1990 s and was the first vacuum biopsy system on the market. In contrast to the devices used for US-guided and MRI-guided interventions, the needle holder of the stereotactic VAB device is equipped with a spring-loading mechanism (Fig. 5.9a). Once the needle has been placed in the holder and advanced to the target position, a vacuum is created by an external vacuum pump connected to the probe by tubing. After the biopsy specimens are sheared from the breast, they are transported inside the needle to the specimen collection chamber and retrieved (Fig. 5.9b). After rotating the needle, the cutting procedure is repeated and the next specimen harvested without having to remove the biopsy needle from the breast.
Vacora system. The Vacora system (Bard Biopsy Systems, Tempe, AZ) is a very compact system that can be used for all image-guided VABs without requiring modification (Fig. 5.10). This system has an internal spring-loading mechanism and vacuum pump. Because the specimens must be retrieved after each sampling by removing the biopsy needle from the breast, a coaxial introduction needle is used. For stereotactic interventions, the system uses a needle guide onto which the coaxial introduction needle is mounted to facilitate the repeated removal and replacement of the biopsy device.
ATEC system. When used for performing a stereotactic biopsy, the ATEC (automated tissue excision and collection) system (Suros Surgical Systems, Inc., Indianapolis, IN) is mounted onto a special spring-loading fixture, which allows the needle to be fired into the breast (Fig. 5.11a, b). Once the needle is placed in the desired position, the biopsy can be performed rapidly by manually rotating the biopsy device after each specimen cutting and retrieving all specimens at the end of the procedure from the specimen collection chamber (Fig. 5.11c). As for the Mammotome device, the vacuum is created by an external vacuum pump.
All VAB units have special fixtures with which they can be mounted onto the different stereotactic biopsy systems.
Procedure
Access path and patient positioning. Before beginning a stereo-tactic VAB, it is necessary to have a mammography examination of the affected breast in two orthogonal views available (Fig. 5.12a–d). Knowing the exact location of the target abnormality guides the procedure. The needle approach, for example, must be chosen such that the target does not lie too close to the proximal skin surface, which might cause problems in creating a vacuum (sample notch not completely in the breast). On the other hand, the target abnormality should not lie too close to the detector/breast support, which could forbid firing the biopsy gun (stroke margin). As a rule, excessively long access paths should be avoided to prevent needle deviation or dislocation of the target lesion.
Fig. 5.9a, b Mammotome system (Ethicon Endo-Surgery, Cincinnati, OH). VAB needle holder for stereotactic biopsy. Cocking lever for the jet mechanism is seen on the side of the system (arrow) (a). Specimen collection window for specimen retrieval (arrow) (b).
Fig. 5.10a–c Vacora stereotactic vacuum biopsy device (Bard Biopsy Systems, Tempe, AZ).
a Special fixture with needle guide for fastening coaxial introduction needle, and gun-carrier for the introduction and removal of the biopsy unit during the procedure.
b Vacora biopsy unit mounted on carrier. Tilt lever at back end of biopsy unit tens the system in place (arrow).
c Specimen retrieval after each cutting process. Release of the specimen into a plastic receptacle protects against blood splattering.
Fig. 5.11a–c ATEC stereotactic vacuum biopsy device (Suros Surgical Systems, Inc., Indianapolis, IN).
a Special fixture with spring mechanism for jet advancement of needle. Cocking lever is seen on the side of the system (arrow).
b ATEC system mounted on carrier. Tubing attachments to external vacuum pump. c Removal of specimen retrieval chamber at the end of completed biopsy procedure.
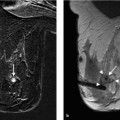
Fig. 5.12a–d Stereotactic biopsy procedure.
a, b Mammography images. Right CC (a). Right LM (b). Microcalcifications in lateral aspect of the breast, approx. 10 o’ clock.
c, d Magnified detail views. e–m See p. 52.
e, f Patient positioning for stereotactic VAB. Positioning in the selected plane for biopsy (e). Positioning of biopsy window and immobilization by applying adequate compression (f).
g Visualization of microcalcifications.
h, i Appropriate placement of cursors in stereo images.
j, k Calibration of coaxial introduction needle length (j) and guidance to calculated x,y position (k).
l, m Local anesthesia and nick incision (l). Coaxial introduction needle in prefire position (m).
n–w See p. 53.
Fig. 5.12n–w
n, o Prefire images.
p Jet-advancement of vacuum biopsy needle and specimen acquisition.
q Specimen radiography.
r, s Postbiopsy images.
t, u Localization coil, placed after biopsy.
v Adhesive strips.
w Mammography in CC projection after biopsy.
The target location on mammography may also make it evident that special patient positioning is necessary to access the abnormality. Target abnormalities very near the lateral aspect of the chest wall, for example, might make it advisable to elevate the contralateral side when positioning the patient on the table.
After analysis of the two-view mammography (CC and ML/LM), the physician and technologist select the appropriate plane for breast compression and decide where to place the biopsy window (Fig. 5.12e, f). If possible, it is preferable to avoid a needle approach in the upper medial quadrant of the breast (scar in cleavage).
Targeting the abnormality. Obtaining a scout image with adequate visualization and centering of the abnormality in the biopsy window is an essential step in the stereotactic procedure (C-arm is perpendicular to the image receptor = 0 °). If the abnormality is not detected in the first scout image, the image must be carefully analyzed to reposition the biopsy window appropriately and then repeat the scout image until a final working image is obtained (Fig. 5.12g). Technically, this process is similar to performing a magnification image in which the abnormality must also be depicted in the partial view. Once an appropriate scout image has been obtained, the next procedure step is to acquire two images by pivoting the C-arm to +15 ° and –15 °, forming the stereo pair for calculation of the target depth. It is then critical to accurately identify and mark the same point in both (planar) stereo images with the cursor (Fig. 5.12 h, i). The unit’s computer then calculates the appropriate coordinates and transfers them to the needle guidance system.
Tips and Tricks
Always compare the abnormality depth with the indicated breast thickness. Make sure that the entire sample notch of the vacuum biopsy needle can be placed within the breast. Rule out a subcutaneous position or position too near the detector/breast support (stroke margin).
Needle placement. The extremely expensive sterile working materials should only be opened after the feasibility of performing the intervention is certain. At this time, the patient can be familiarized with the noises she will be hearing during the examination (needle firing, vacuum, and cutting noises). Before beginning the examination on the Lorad stereotactic table, the needle is placed in the needle guide for calibration. After positioning the needle tip in line with the notch and bead sight located in the upper part of the compression plate, calibration is performed (Fig. 5.12j). The needle can then be moved to the calculated target coordinates in the x- and y-axes (Fig. 5.12k). The needle is carefully advanced toward the skin to indicate the site of needle insertion for disinfection and administration of local anesthesia. Once anesthesia is effected, a small skin incision of approx. 3–5 mm is made to allow smooth passage of the biopsy needle (Fig. 5.12l). The biopsy needle is then advanced to the appropriate depth (Fig. 5.12 m). Some stereotactic systems signal if the stroke margin falls below the tolerance value when advancing the needle to the appropriate z-coordinate value.
Tips and Tricks
An adequate skin incision of 3–5 mm is important to allow smooth needle advancement and avoid skin drag, which could displace breast tissues and the target lesion.
Prefire stereo images (+15 ° and –15 °) are acquired to ensure correct needle placement and to check for abnormality movement (Fig. 5.12n, o). The anterior needle guide should be retracted to prevent superimposition over the area of interest. If the needle position is not correct, the error must be analyzed. If necessary, the target can be marked anew on the last stereo pair, and the coordinate calculations redone.
Sample harvesting. The trigger button is pushed to fire the needle forward only after ensuring that the prefire needle position is correct, the stroke margin is positive, and the anterior needle guide is forward. The direction in which the first samples are harvested is sometimes influenced by the position of the target lesion in relation to the needle. Generally, the sample notch is rotated in the closed position to acquire contiguous samples: e. g., 12, 1, 2, 3, to 11 o’clock. An alternate approach (our philosophy) is 12, 2, 4, 6, 8, and 10 o’clock, then 1, 3, 5, 7, 9, and 11 o’clock (Fig. 5.12p). The harvesting of tissue samples normally causes little discomfort. Additional anesthetic can be given through the biopsy or coaxial introduction needle should a patient experience pain.
Good Practice Recommendations*
VABs typically use 11- to 9-gauge needles.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree