and Ashutosh Dash2
(1)
Nuclear Security and Isotope Division, Oak Ridge National Laboratory, OAK RIDGE, USA
(2)
Isotope Production and Applications Division, Bhabha Atomic Research Centre, Mumbai, India
2.1 Introduction
Unsealed radioactive sources are administered by certified medical staff, and the use of 131I-iodide for ablation of thyroid tissue and surgical remnants is the classic example in endocrinology. While application of sealed radioactive sources such as 192Ir in radiation oncology generally involves the use of afterloading devices, for instance, unsealed radioactive sources for the selective delivery of radiation to target tissues or organs without significantly affecting normal tissues are known as radionuclide therapy and are practiced by nuclear medicine specialists. This promising treatment option has been available for more than 70 years but had been for many years confined for radiation therapy of limited diseases, with the treatment of thyroid cancer the most prominent example.
Over the last 40–50 years, rapid and widespread interest in radionuclide therapy has been the driving force behind extensive research focused on the design, development, testing, and clinical evaluation of many novel radiopharmaceuticals. Production of radionuclides which are required for radionuclide therapy (Chaps. 5, 6, 7, and 8) is the first step in the development of therapeutic radiopharmaceuticals and also the most crucial aspect for the success as well as sustainable growth of radionuclide therapy. Development of production and processing technologies has been an area of intense research since there are a variety of therapeutic radionuclides which differ based on their physical half-lives, type of particle emission (β−, σ, Auger), particle emission energy, specific activity, and chemistry (Ehrhardt et al. 1998; Karenlin and Toporov 1998; IAEA 2012; Mausner et al. 1988; Neves et al. 2005; Qaim 2012; Ruth et al. 1989; Troutner 1987; Volkert et al. 1991; Yeong et al. 2014; Zhuikov 2014). While the production of therapeutic radionuclides requires a thorough understanding of the nuclear reactions and decay schemes, development and implementation of processing methods require knowledge of the appropriate aqueous solution chemistry. This chapter discusses the key aspects related to the production of important therapeutic radionuclides, embracing current and possible future needs in the rapidly advancing field of radionuclide therapy. While both research reactors and accelerators are the principal technologies which offer the possibility for sustainable production of a wide range of radionuclides, there are several issues and challenges that must be considered in order to fully realize their potential (Ehrhardt et al. 1998; IAEA 2012; Mausner et al. 1988; Neves et al. 2005; Qaim 2012; Ruth et al. 1989; Troutner 1987; Volkert et al. 1991; Yeong et al. 2014; Zhuikov 2014). Depending on both the demands and applications, efficient production of therapeutic radionuclides plays a key role for current opportunities in technology development and to fulfill future research needs for radionuclides of emerging interest that appear promising for radionuclide therapy.
Radionuclide therapy utilizes unsealed radioactive sources designed to deliver therapeutic doses of ionizing radiation to specific disease sites and is an attractive treatment option either for curative intent or for disease control and palliation (Chatal and Hoefnagel 1999; Joensuu and Tenhunen 1999). This methodology combines the advantages of target selectivity similar to that of brachytherapy or external beam radiotherapy and at the same time is systemically similar to chemotherapy. Systemic administration strategies offer the prospect of treating disseminated metastatic tumors (McDougall 2000; Volkert and Hoffman 1999). The basic principle primarily underlines the administration of a radiopharmaceutical for selective accumulation at the diseased tissue either to ablate or damage cells through the emission of energetic β−-particles, α-particles, or Auger electrons (e−) (Britton 1997; Heeg and Jurisson 1999; McEwan 1997; Serafini 1994). The targeting feature of radionuclide therapy is achieved either by the intrinsic targeting properties of some radionuclides or by conjugating the radionuclide to specific carrier molecules. Therapeutic radiopharmaceuticals generally consist of two entities, which include the radionuclide, which is the radiation source, and a targeting moiety that determines tissue localization. In light of the explicit need to deliver sufficient cytotoxic radiation to the target cells while not causing unmanageable side effects, it is imperative that the radiopharmaceutical accumulates selectively in the diseased tissue, clears rapidly from the blood and other normal organs, and has rapid transit through the excretory organs, in order to minimize radiation damage to normal tissues.
Over the years, radionuclide therapy has undergone rapid and continual evolutionary cycles, relying on empirically prepared radiopharmaceuticals and presently progressing to the use of intelligent design of specific agents for targeted therapy. As the development of therapeutic radiopharmaceuticals has progressed, the use of the metallic radionuclides as well as novel target-specific pathways is now a hallmark (Spencer et al. 1987; Hoefnagel 1991; Stöcklin et al. 1995; O’Donoghue et al. 1995; Ercan and Caglar 2000; Srivastava 1996a, b; Mausner and Srivastava 1993; Srivastava and Dadachova 2001; Cuaron et al. 2009; Tolmachev et al. 2004; Unak et al. 2002; Stanciu 2012; Srivastava and Dadachova 2001; Das and Pillai 2013). A number of metallic radionuclides have been combined with key targeting macromolecules, which include whole antibodies, antibody fragments, small peptides, peptidomimetics, or nonpeptide receptor ligands, and have been studied for their potential use in tumor therapy. The use of metallic radionuclides is attractive and offers the convenience of designing a broad spectrum of exotic radiopharmaceuticals by modifying the coordination environment which surrounds the metal. These advances have thus led to considerable fascinating research and innovative strategies for treating disseminated human malignancies and various other disorders.
In principle, to achieve desired therapeutic effects, the radionuclide chosen should exhibit the necessary physical, chemical, and biological properties. A wide range of radionuclides based on their physical half-lives, energy of particle emission, type of particle emission and specific activity, etc., are available which can be used for the treatment, control, and palliation of many disseminated diseases. Although a summary is not provided here, subsequent chapters provide detailed information describing the properties of key therapeutic radioisotopes of current interest (see Chaps. 3, 4, 5, and 6). The chronology includes selection of a radionuclide with apparent optimal properties for a specific application with subsequent development of methods for routine production, processing, and purification.
A variety of therapeutic radionuclides are now regularly commercially produced as well as at many nuclear science research centers, utilizing research reactors and accelerators. In view of the tremendous prospects associated with the production of therapeutic radionuclide, a number of excellent review articles have been published (Cutler et al. 2013; Ruth et al. 1989, Volkert et al. 1991; Ehrhardt et al. 1998; Karenlin and Toporov 1998; IAEA 2012; Knapp et al. 1998; Mausner et al. 1988; Qaim 2012; Neves et al. 2002, 2005; Qaim and Coenen 2005; Karelin et al. 2000; Troutner 1987; Yeong et al. 2014; Zhuikov 2014). The rate at which interest and production methods are developing for a variety of radionuclides appears to be increasing. In order to sustain and broaden the scope and promote further development of radionuclide therapy, radionuclide production and processing strategies need a vision for sustained future growth. Taking account of the progress made in therapeutic radionuclide production, it is timely to provide up-to-date information for current and expected activities in this field. The goal of this chapter is to present a comprehensive overview of production technologies and separation methodologies for several radionuclides of current interest and for those which have considerable potential for future applications in radionuclide therapy.
2.2 Criteria for Selection of Therapeutic Radionuclides
The success of radionuclide therapy in the nuclear medicine arena is dependent on the selection of appropriate radionuclides (Volkert and Hoffman 1999; Srivastava 1996a; Volkert et al. 1991). Although numerous radionuclides have potential applications in radionuclide therapy, only a very few possess favorable nuclear, physical, and biological characteristics which would identify them as practical for clinical use. The ultimate choice is based on several factors described in the following sections.
2.2.1 Particle Emission
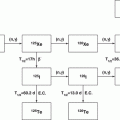
Radionuclides which are suitable for radionuclide therapy owing to their high linear energy transfer (LET) to deliver localized cytotoxic ionizing radiation include those which decay by beta-particle emission (β−), alpha-particle emission (α), and radionuclides that decay by electron capture (EC) and internal conversion (IC), leading to the emission of Auger and Coster–Kronig (C–K) electrons. The choice of a particular radionuclide is strongly dependent on the LET value and tissue range of emissions. Each type of particle emitted by the radionuclide has a different range, effective distance (see Fig. 2.1, Chap. 12), and relative biological effectiveness (RBE). The size of the tumor or the tissue mass to be treated should be matched with the appropriate effective radiation range. Radionuclides emitting beta particles are effective for large tumors owing to their large millimeter range and cross fire. Since the range of α−particles is confined to 50–100 μm, they are effective for small tumors and micrometastases. Radionuclides that emit Auger and C–K electrons are effective for only single cells when they across the cell membrane. Nuclear targeting to induce irreversible DNA damage is the goal for Auger therapy, although the established efficacy of some radiopharmaceuticals labeled with Auger emitters such as 111In-octreotate, which do not reach the cell nucleus, has not yet been elucidated (Table 2.1).
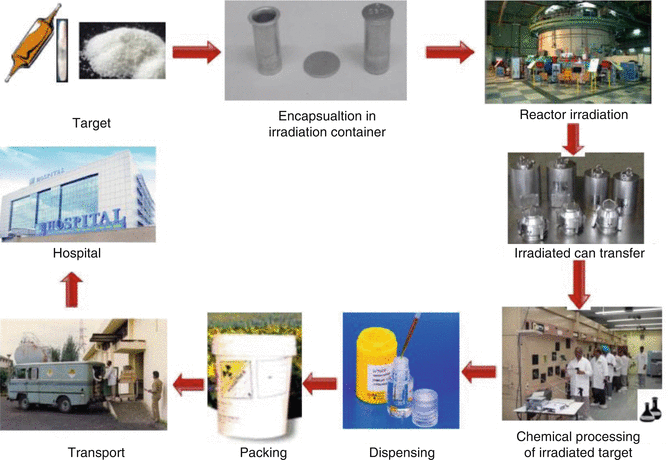

Fig. 2.1
Various steps of the therapeutic radionuclide production cycle
Table 2.1
General characteristics of therapeutic radionuclides
Decay | Particles (#)a | E (min)–E (max) | Range | LET (KeV/μm) |
|---|---|---|---|---|
α++-particle | He nuclei (1) | 5–9 MeVb | 40–100 μm | ~80 |
β−-particle | Energetic electrons (1) | 50–2300 keVc | 0.05–12 mm | ~0.2 |
EC/IC | Nonenergetic electrons (5–30) | eV–keVb | 2–500 nm | ~4–26 |
2.2.2 Tissue Treatment Morphology
The nature of the particle emission required largely depends on the size of the tumor or tissue treatment site, tissue distribution pharmacokinetics of the tracer, and other factors which are important to maximize therapeutic effectiveness.
2.2.3 Radionuclide Half-Life
The radionuclide physical half-life should be well matched with the in vivo biolocalization and clearance properties of the radiolabeled compound. If the half-life is too short, radionuclide depletion resulting from radioactive decay could occur before the radioisotope reaches and has sufficient residence time at the targeted site. Conversely, a long treatment site residence time could cause unnecessary radiation dose to normal tissues. A physical half-life of 1–14 days is often felt to be the optimal half-life range. In light of the explicit need to prepare site-specific radiolabeled compounds of high specific activity to target low-capacity systems such as receptors or antigens, the specific activity of the radionuclide should be high, and for this reason, no-carrier-added (NCA) radionuclides are preferable for these applications.
2.2.4 Radionuclide Decay Products
Ideally, the daughter radionuclide decay product should be stable (nonradioactive) or decay with low-energy emissions and have a short half-life.
2.2.5 Radionuclide Purity
The availability of radionuclides with the highest standard of purity (radionuclidic, radiochemical, elemental, and chemical) is essential for clinical applications for radionuclide therapy. These high purity requirements necessitate the use of nuclear reactions as well as chemical separation and purification schemes that result in products with high radionuclidic purity. To this end, enriched stable target nuclides are most often desirable to preclude ancillary activation of other isotopes in target.
2.2.6 Gamma Emissions
Emission of gamma rays in useful abundance and with optimal energy is usually very advantageous to permit quantification of targeted uptake and biokinetics to allow low-dose imaging, especially during staging, for dosimetry estimates and for monitoring response to therapy. Although gamma radiation with low abundance is useful for imaging purposes, high abundance as well as high-energy gamma radiation results in poor images and contributes significantly to the whole-body radiation burden of the patient under treatment without significantly increasing the radiation damage to the target tissue.
2.2.7 Radiolabeling Chemistry
The radionuclide chemical properties should permit the opportunity for introduction into a wide range of targeting molecules.
2.2.8 Economic Factors
For projection of widespread and cost-effective dependable availability, the commercial production of the required activity levels of radionuclides with high specific activity and purity is an important aspect for radiopharmaceutical development, and for these reasons, the production of radionuclides is discussed in detail in Chaps. 5, 6, 7, and 8.
Although only a limited number of radionuclides that satisfy the above eight criteria have been found useful in designing therapeutic radiopharmaceuticals, emerging interest is focused on a number of promising radionuclides that may have future importance for radionuclide therapy. The issue of their current and future availability for radionuclide therapy studies warrants continued investigation. A wide range of radionuclides of different radiation emission characteristics, radiobiological effectiveness, and range of action have been used in the treatment of different diseases. The radionuclidic physical and nuclear characteristics must be appropriately matched with the therapeutic application of interest, or the disease under treatment, as discussed below.
2.3 Beta-Particle-Emitting Radionuclides
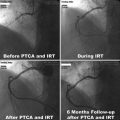
Radionuclides that decay by β−-particle emission have maximum kinetic energies of 0.3–2.3 MeV with corresponding ranges of ~0.5–12 mm in soft tissue (Fig. 2.1, Chap. 12). Such long penetration range precludes the need for cellular internalization and can be effectively targeted at or near to the cell membrane. The range of β−-particles, as compared to cell diameter, permits β−-particles to traverse several cells (10–1000), a useful therapeutic property that has been termed “crossfire,” which ensures sufficient dose delivery to each cell in a large tissue mass. Depending on the size and location of the tissue site for dose delivery, the choice of the β−-emitter may be different. Although β−-particle-emitting radionuclides are effective for medium to large size tumors (Chaps. 9 and 10), for instance, they are less effective in treating smaller metastatic tumors owing to deposition of a larger fraction of the particle energy outside the tumor volume. On the other hand, when the tumor is large in comparison to the range of β−-particle, most of the energy is deposited within the tumor. Various other important clinical applications of β−-emitting radioisotopes are described for bone pain palliation (Chap. 12), nonmelanoma skin cancer therapy (Chap. 13), treatment of arthritis (synovectomy, Chap. 14), and arterial restenosis therapy (Chap. 15).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree