1 Acute Ischemic Stroke
1.1 Hyperdense Vessel Sign
1.1.1 Clinical Case
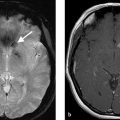
A 23-year-old female, who woke up with aphasia and right-sided hemiparesis following a cardiac surgery (Fig. 1.1).

1.1.2 Description of Imaging Findings and Diagnosis
Diagnosis
Left M1 occlusion with hyperdense artery sign.
1.1.3 Background
Non-contrast CT is the primary modality in acute ischemic stroke imaging at a vast majority of centers. Because of this, understanding the imaging manifestations of acute ischemic stroke is essential for radiologists, neurologists, and other neuro vascular specialists. Because acute stroke, secondary to large vessel occlusion is the most devastating type of acute ischemic stroke, the prompt identification of imaging manifestations of large vessel occlusion is essential. The hyperdense artery sign is a well-described imaging manifestation of large vessel occlusion. In some centers, a hyperdense artery sign in the setting of a clinical severe stroke and a good Alberta stroke program early CT score (ASPECTS) will prompt endovascular intervention, even without a CT angiogram or CT perfusion study.
1.1.4 Imaging Findings
When evaluating the presence of a hyperdense vessel sign on non-contrast CT, the use of thin-slice (<2.5 mm) CT is strongly recommended. Many centers have shifted to using 1-mm slice thickness for all stroke protocols as it allows for the excellent delineation of the cerebral vasculature and has been shown to be substantially more sensitive and specific in identifying the hyperdense artery sign. The use of thicker slice CT (i.e., 3 mm or larger) will result in partial volume averaging of the horizontally traversing MCA branches due to their diameter being typically 3 mm or less (Fig. 1.2). Thin-slice CT also allows for a more accurate evaluation of thrombus density, thrombus location, and thrombus length-variables, which have been shown to be correlated with angiographic outcomes after mechanical thrombectomy. It is particularly helpful in identifying distal MCA occlusions, particularly branches which course along the insula (Fig. 1.3).
Studies examining the association between non-contrast CT findings and clot composition have been very consistent in demonstrating that the proportion of RBCs in a clot is strongly correlated with clot density. Meanwhile, fibrin-rich clots are known to be low density on non-contrast CT. Because roughly 70–80% of clots are RBC rich, in approximately 70–80% of large vessel occlusion cases, there will be a hyperdense artery sign. It is important to point out that the absence of a hyperdense artery sign does not mean the absence of intravascular thrombus or large vessel occlusion (Fig. 1.4).



There are pitfalls of the hyperdense artery sign as an imaging marker of a large vessel occlusion. First, the density of blood in the vessels is strongly correlated with hematocrit. This explains why RBC-rich clots are more hyperdense than fibrin-rich clots. However, patients with a high hematocrit will have diffusely hyperdense arteries. Thus, looking for symmetry in vascular attenuation between the affected hemisphere and the non-affected hemisphere is important. When using thicker slices of CT, the partial volume averaging of vascular calcifications can result in the appearance of a hyperdense artery sign. This can be potentially mitigated by using a thinner-slice CT protocol. The hyperdense artery sign is not reliable in patients who have had recent contrast administration (i.e., post-cardiac catheterization strokes). Lastly, it is essential to point out again that a fibrin-rich clot will not be dense on CT and these lesions make up roughly 20% of clots in the setting of large vessel occlusion.
CT angiography (also using thin-section CT) is very useful in clot imaging. Identification of a contrast gap between the proximal and distal end of the clot can help to assess clot length and thus aid in device choice, especially stent retriever length. Delayed-phase CTA is particularly useful in assessing clot burden and thrombus length as it can take time from contrast to make it to the distal face of the clot. There has been growing interest in the assessment of clot perviousness/permeability to iodinated contrast as increasing density/enhancement of clot on CTA has been shown to be associated with revascularization outcomes.
1.1.5 What the Clinician Needs to Know
Presence or absence of hyperdense artery sign
Location and estimated length of the thrombus
1.1.6 High-Yield Facts
The hyperdense artery sign is a sensitive and specific marker for large vessel occlusion in the setting of acute ischemic stroke.
Pitfalls for the hyperdense artery sign include high hematocrit, recent contrast administration, and partial volume averaging in the setting of a calcified vessel.
Thin-slice CTA with delayed phase imaging is an important tool in characterizing clot location and extent.
Further Reading
[1] Ahn SH, Choo IS, Hong R, et al. Hyperdense arterial sign reflects the proportion of red blood cells in the thromboemboli of acute stroke patients. Cerebrovasc Dis. 2012; 33:236 [2] Riedel CH, Zoubie J, Ulmer S, Gierthmuehlen J, Jansen O. Thin-slice reconstructions of nonenhanced CT images allow for detection of thrombus in acute stroke. Stroke. 2012; 43(9):2319–2323 [3] Borst J, Berkhemer OA, Santos EMM, et al. MR CLEAN investigators. Value of thrombus ct characteristics in patients with acute ischemic stroke. AJNR Am J euroradiol. 2017; 38(9):1758–17641.2 ASPECTS Score and CT Signs of Early Ischemia
1.2.1 Clinical Case
An 83-year-old male with acute onset right arm weakness, right facial droop, and aphasia (Fig. 1.5).

1.2.2 Description of Imaging Findings and Diagnosis
Diagnosis
Left M1 occlusion with hyperdense artery sign. ASPECTS score of 7 with infarction affecting the left insula, lentiform nucleus, and caudate.
1.2.3 Background
As mentioned previously, non-contrast CT is the workhorse in acute stroke imaging. When patients present with stroke-like symptoms, the three most important tasks the radiologist must accomplish are: (1) rule out an intracranial hemorrhage or any other imaging findings that would contraindicate thrombolysis; (2) characterize the extent of infarction using the ASPECTS score; and (3) identify the presence or absence of a hyperdense artery sign.
The ASPECTS score has been shown to be a reproducible tool in assessing the extent of infarction in the setting of acute ischemic stroke affecting the internal carotid artery and middle cerebral artery territories, the locations for the vast majority of large vessel occlusions. It is important to note that it does not apply to the posterior circulation or the anterior cerebral artery (ACA) territory. Most of the recently published randomized controlled trials on mechanical thrombectomy for acute ischemic stroke used an ASPECTS score of ≥6 as the part of their inclusion criteria. In general, patients with ASPECTS scores of 5 or lower have been shown to have a very poor prognosis regardless of successful intervention; however, this issue is still up for debate. The data are clear however that the higher the ASPECTS score, the better the chance for a meaningful neurological recovery in the setting of successful revascularization.
There has been growing interest in developing reliable and reproducible tools for assessing infarct extent in the posterior circulation and correlating this to patient prognosis. To date, there is no tool that has been widely accepted. One reasonable imaging scale is the posterior circulation ASPECTS (pc-ASPECTS) that uses CT angiographic source images to assess infarct extent. A pc-ASPECTS on CTA source images of <8 has been shown to be strongly correlated with poor functional outcomes.
1.2.4 Imaging Findings
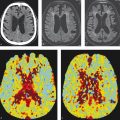
The ASPECTS score is a 10-point grading scale for identifying the extent of infarcted brain in the setting of acute ischemic stroke affecting the ICA and MCA territories. The territories of the ASPECTS score are shown in Fig. 1.6 and include the lentiform nucleus, caudate head/body/tail, internal capsule, and insula. At the level of the basal, ganglia are the M1-M3 territories, which include the anterior MCA cortex corresponding to the frontal operculum (M1), MCA cortex lateral to the insular ribbon corresponding to the anterior temporal lobe (M2), and posterior MCA cortex corresponding to the posterior temporal lobe (M3). At the level of the ventricles immediately above the basal, ganglia are the M4-M6 territories, which include the anterior MCA territory immediately superior to M1 (M4), lateral MCA territory immediately superior to M2 (M5), and posterior MCA territory immediately superior to M3 (M6). For each of the 10 above-listed territories, one point is subtracted, if the grey-white matter differentiation is attenuated in this territory. The ASPECTS score is thus scored as 10(number of affected territories).
When assessing the ASPECTS score, it is important to narrow your windows width and levels to so-called “stroke windows.” The ideal window width and levels vary by study and center; however, many neuroradiologists prefer reviewing acute stroke CTs using WW of 50 and WL of 30. Ultimately, the goal is to window and level the CT in order to maximize gray-white differentiation to allow for more easy identification of infarcted brain (Fig. 1.7).
There are pitfalls to the assessment of ASPECTS score. Patient motion may severely affect the interpretation of ASPECTS, particularly in the MCA cortices. Old infarctions and leukoaraiosis should not be counted as part of the ASPECTS score; however, they can confound one’s ability to properly assess gray-white differentiation and in such cases evaluating the presence or absence of parenchymal enhancement on CTA source images may be helpful.
There are a number of basic stroke imaging signs that the radiologist or other neurovascular specialist should be keenly aware of. In the setting of acute ischemic stroke, there is often gaze deviation toward the side of the stroke due to impairment of the frontal eye fields (Fig. 1.8). Thus, a patient with a right MCA stroke often has gaze deviation to the right. Loss of the insular ribbon (Insular Ribbon Sign) is another common early finding and included in the ASPECTS score. The presence of hyperdense arteries or calcified emboli, as discussed in the previous (Chapter 1.1), is important imaging manifestations of stroke as well.



Non-contrast CT is not as reliable for the posterior circulation due to the presence of beam hardening artifact. Posterior circulation structures include the thalami, cerebellar hemispheres, posterior cerebral artery territories (occipital lobes and inferior/mesial temporal lobes), and midbrain and pons. In the pc-ASPECTS score, the midbrain and pons are each given 2 points, whereas all other structures are given 1 point. Because non-contrast CT is not as helpful due to artefactual findings, evaluating the presence or absence of diminished contrast enhancement in affected territories on CTA is recommended when assessing the extent of ischemia in basilar artery occlusions.
1.2.5 What the Clinician Needs to Know
ASPECTS score including the location of all areas with affected ischemia
Presence of hyperdense artery sign
Other imaging manifestations of acute stroke including gaze deviation
Presence or absence of intracranial hemorrhage
1.2.6 High-Yield Facts
ASPECTS score is the gold standard in CT assessment of ischemic injury to the MCA territory.
Pitfalls include motion artifact, old infarct, and chronic white matter ischemic changes. Using source images of CTA is helpful in assessing the extent of ischemia in such cases.
Assessing the extent of ischemia in the posterior circulation is difficult due to beam hardening artifact from skull base structures.
Using narrow window widths and levels (50/30) allows for improved assessment of ASPECTS score.
Further Reading
[1] Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000; 355(9216):1670–1674 [2] Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001; 22(8):1534–15421.3 CT Angiography in Acute Stroke including Imaging of Collateral Supply
1.3.1 Clinical Case
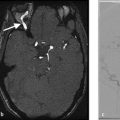
A 56-year-old male with acute onset left arm and leg weakness, dysarthria (Fig. 1.9).

1.3.2 Description of Imaging Findings and Diagnosis
Diagnosis
Right M2 branch occlusion with hyperdense artery sign in the right insula. ASPECTS score is 9 with infarction affecting the right insula. Branch occlusion is best appreciated on coronal MPR. Excellent collaterals.
1.3.3 Background
CT angiography is the workhorse of intracranial and extracranial vascular imaging in the emergency setting. CT angiography is essential in the setting of acute ischemic stroke as it allows for evaluating the patency of the cervical and intracranial vasculature, aortic arch anatomy, and the presence of collaterals.
There has been much emphasis as of late on imaging of collateral artery supply in acute ischemic stroke. There are two types of collaterals, primary, and secondary. Primary collaterals include large vessels such as the anterior communicating and posterior communicating arteries. Secondary collaterals are primarily leptomeningeal collaterals. Leptomeningeal collaterals are pial anastomotic branches joining the terminal cortical branches of major cerebral arteries (i.e., middle, anterior, and posterior cerebral arteries) along the surface of the brain. These vessels are dormant under normal circumstances but can be recruited in the setting of a chronic or acute occlusion. Some degree of leptomeningeal collaterals can be seen in approximately 80% of patients. The longevity and robustness of these collaterals is however limited. There is also wide variability in the distribution, size, and number of collaterals between patients. The robustness of one’s collaterals has been found to be strongly correlated with clinical outcomes.
1.3.4 Imaging Findings
CTA is invaluable in the setting of acute ischemic stroke. The most important information obtained on CTA for acute ischemic stroke is the presence or absence of a vascular occlusion that can be identified as a focal filling defect in the artery. There are a number of useful tips and techniques for maximizing one’s ability to detect such an occlusion, particularly when the occlusion is located beyond the M1 segment.
When interpreting a CTA, it is important to look at both source images (thinner cuts) and the MIP images. Multiplanar reformats are constructed with maximum intensity projection images, which have a slice thickness of 6.5 mm, at every 2.5 mm. Source images are typically between 0.5 and 0.75 mm thick. In most cases, the axial MIP images are sufficient for identifying a large vessel occlusion. However, evaluating thin-cut images substantially increases the sensitivity and specificity for the detection of both proximal and distal branch occlusions. In fact, if MIP images are read in isolation, the sensitivity and specificity for the detection of a vascular abnormality are only 70 and 50%, respectively. These values increase to 90 and 80%, respectively, when axially reformatted source images are also interrogated. Multiplanar reformats in the coronal plane are extremely useful in identifying M2 branch occlusions as well as the patency of insular branches. Multiplanar reformats in the sagittal plane are helpful in identifying patency of distal M2/M3 branches as they course along the insula. When an occlusion is not obvious, a careful interrogation of sagittal and coronal reconstructed images is essential. Optimal window settings for interpreting a CTA are in the neighborhood of WW 600 and WL 200 (Fig. 1.9).
When evaluating a CTA in the setting of acute ischemic stroke, there are a number of other important imaging findings one should report. Reporting the presence of a bovine arch, left vertebral artery origin directly from the arch or a complex Type 3 aortic arch is very helpful as it can influence the neurointerventionalist’s choice or route of access. The presence of substantial arch atheromatous disease or an aortic dissection can provide clues regarding stroke origin (Fig. 1.10). The patency and tortuosity of the cervical vasculature should also be evaluated. Large stenotic atheromatous plaques or dissections ipsilateral to the ischemic territory can alter endovascular management strategies and provide information regarding stroke origin. Substantial cervical vascular tortuosity may change management strategies as well.
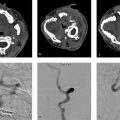
The evaluation of cerebral blood flow using CT perfusion, single-phase CTA, and multiphase CTA have all been proposed as tools that can be used to identify collaterals in patient selection for acute ischemic stroke treatment. Examples of patients with good and poor collaterals is provided in Fig. 1.11. Grading scores using single phase and multiphase CTA are typically subjective with the assessment of collaterals being a rough estimate of the proportion of the affected vascular territory in which the distal vessels are opacified. These collateral grading scores are used primarily for research purposes but familiarity with their general concepts is important to refine patient selection for stroke thrombectomy. The Christoforidis and Miteff collateral scores use single phase CTA while the ASPECTS and modified American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) scores use multiphase CTA (Table 1.1). In multiphase CTA, images are acquired during peak arterial, peak venous, and late venous phases. By acquiring temporal information at three time points, multiphase CTA allows for a dynamic assessment of pial collateral filling. Multiphase CTA has been shown to reliably predict clinical outcomes in patients with acute ischemic stroke. Delayed phases also allow for a more accurate assessment of thrombus length. Another advantage of multiphase CTA is the fact that it does not require any mathematical algorithms or complex post-processing tools like CT perfusion.
There are a number of pitfalls to both single-phase and multiphase CTA. For single-phase CTA, a common pitfall is the so-called pseudoocclusion (Fig. 1.12). In the setting of an ICA terminus occlusion, there is poor antegrade flow in the cervical ICA. Because of this, the cervical ICA is often not opacified or poorly opacified. This results in the appearance of a cervical ICA occlusion. Delayed phase imaging can be helpful in sorting out the presence or absence of a pseudoocclusion by allowing enough time for contrast to reach the mid and distal cervical ICA. The presence of a proximal flow limiting stenosis can affect contrast opacification on both single and multiphase CTA as can the presence of poor cardiac output.



Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree