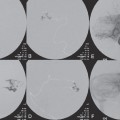
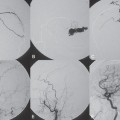
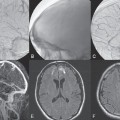
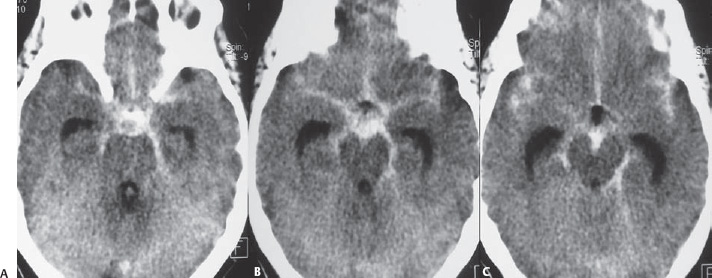
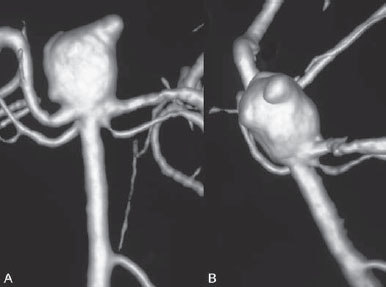
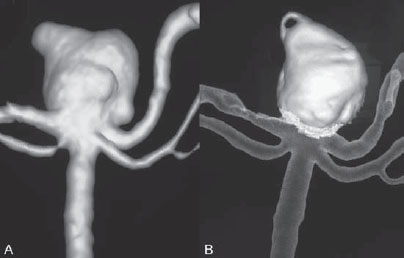
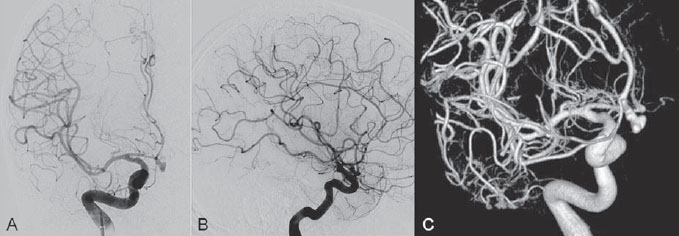
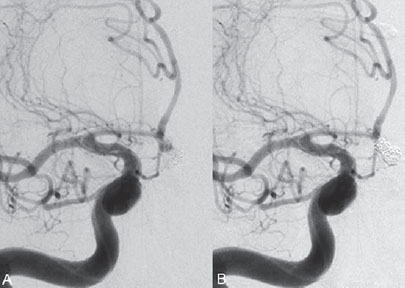
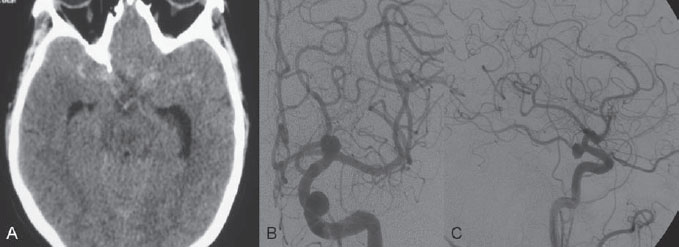
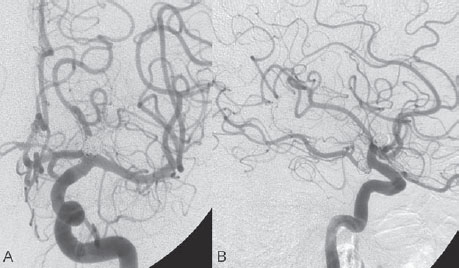
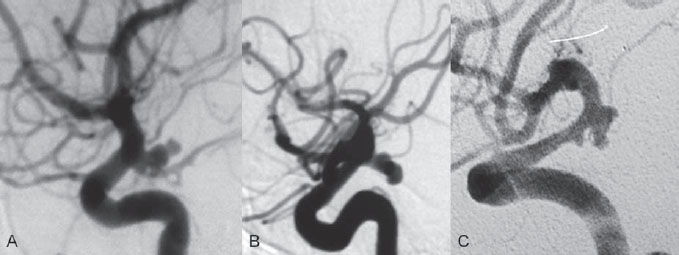
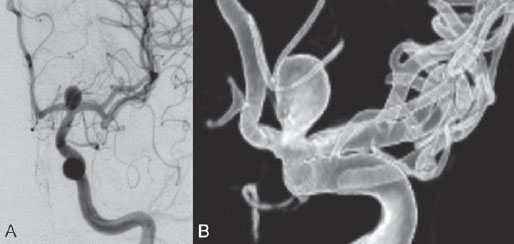
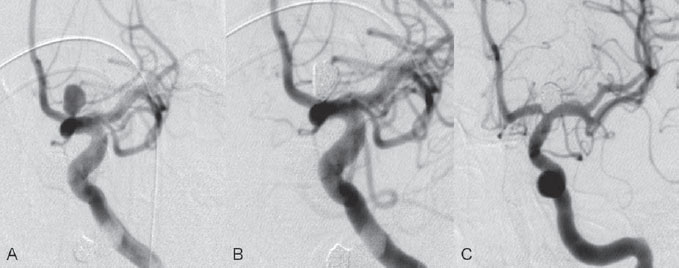
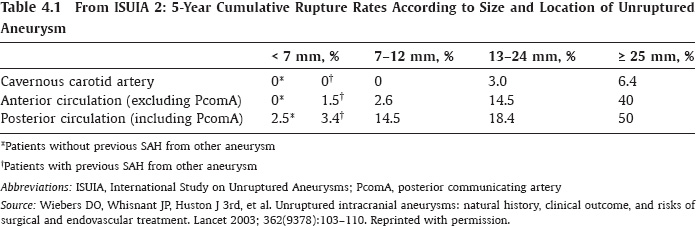
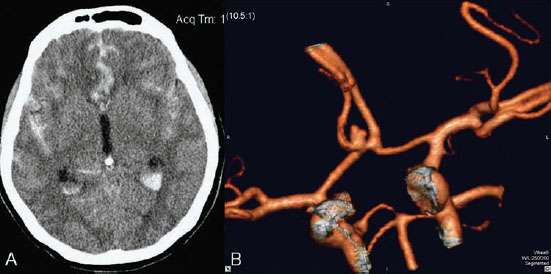
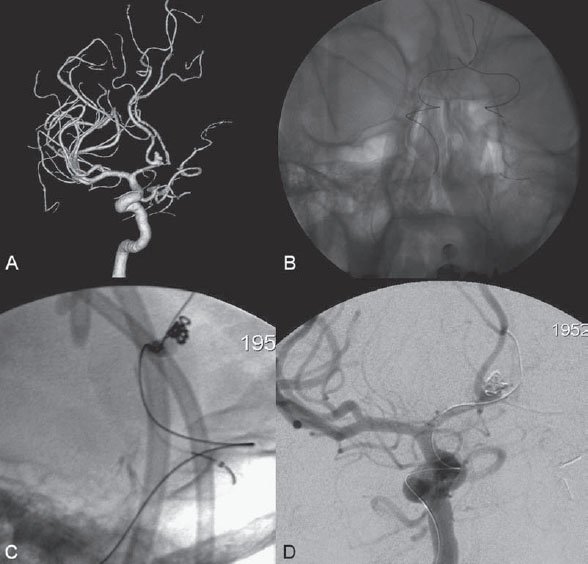
PART 1 Aneurysms and Subarachnoid Hemorrhage A 54-year-old man presents with the sudden acute onset of headache, nausea, and vomiting. When he arrives in the emergency department, he has a decreased level of consciousness and is not fully oriented. Emergency CT is performed. Fig. 1.1 (A–C) Unenhanced axial CT reveals nearly symmetric hyperdensity within the basal cisterns, corresponding to diffuse SAH. Prominent size of the temporal horns of the lateral ventricles is noted. Note the small round hypodense area within the hyperdense clot at the level of the prepontine cistern, suspicious for a basilar tip aneurysm. CT CT demonstrated a basal subarachnoid hemorrhage (SAH) with a nearly symmetric distribution, a small blood clot in the fourth ventricle, and no evidence of intraparenchymal hemorrhage. Enlargement of the ventricles (especially the temporal horns) was noted. Within the prepontine cistern, a focal round area without subarachnoid blood was seen (Fig. 1.1). CTA confirmed the suspicion of a single basilar tip aneurysm, and angiography with the intention to treat was performed during the same session. DSA Injection of the left vertebral artery demonstrated a basilar tip aneurysm. 3D rotational angiography revealed an irregular shape with a cranial daughter aneurysm (or bleb), which was regarded as the probable point of rupture. The aneurysm size was 12 × 10 × 9 mm. The neck of the aneurysm was considered to be moderately wide (5 mm). A cranial fusion-type of basilar apex was present, and the most proximal portions of both P1 segments were incorporated in the neck. No focal vasospasm was seen (Fig. 1.2) Ruptured aneurysm of the basilar tip The 6F multipurpose catheter was placed in the distal vertebral artery. Control injections demonstrated good flow and no vasospasm surrounding the guiding catheter. Following a roadmap in the working projection, the microcatheter was advanced via the micro-guidewire and carefully placed in the lower one-third of the aneurysm. Here, the first coils were deployed, with care taken not to advance the coils into the cranial daughter aneurysm. With the first three complex-shaped coils, a stable frame for subsequent filling of the aneurysm could be established. Control runs demonstrated no protrusion of coils into the parent vessels and good framing of the aneurysm neck. Subsequently, the microcatheter was advanced as far distally into the aneurysm as possible to continue coiling from the dome to the neck with 2D coils, which allowed dense packing of the aneurysm with only two coil loops (of smaller, soft coils) protruding into the daughter aneurysm. If protrusion beyond the frame was noted caudally, the coils were repositioned. A control run demonstrated complete exclusion from the aneurysm and a dense coil mesh (Fig. 1.3) No thromboembolic complications were noted, and all distal vessels were well preserved. The procedure was carried out with the patient on heparin (5000 IE), which was not reversed following the procedure. Saccular aneurysms are the most frequently encountered form of aneurysm. They arise at arterial bifurcations and resemble berry-like outpouchings of the vessel wall. Their etiology and pathophysiology are complex and poorly understood. Although genetic factors likely contribute to their development, other factors, such as aging, elevated blood pressure, blood shear stresses, and cigarette smoking, are also thought to contribute to the formation and/or rupture of aneurysms. The average annual risk for SAH is 10 to 21 per 100,000 people, with higher incidence rates encountered in Finland and Japan. Risk factors for hemorrhage include female gender, increasing age, previous symptomatic aneurysm, larger size, and location in the posterior circulation. The prognosis following untreated aneurysmal SAH is poor; in a community-based study, the mortality rates were 12% before patients reached the hospital, 43% within the first week, and 56% at day 30 if the aneurysm was left untreated. In a meta-analysis of untreated ruptured aneurysms performed by Heros in 1990, significant morbidity and mortality after the first hemorrhage were estimated to be 50%, with a further mortality of 35% after the second bleed. The risk for rebleeding without treatment is ~1% per day for the first 9 days, increases to 4% at day 10, then fluctuates at ~ 2 to 3% per day during days 11 through 15. The cumulative risk for rebleeding is ~20% in the first 2 weeks and 30 to 50% in 6 months. Aneurysms typically arise at branch points on the parent artery. The branch point may be formed by the origin of a side branch from the parent artery (e.g., posterior communicating artery) or by subdivision of a main arterial trunk into two trunks (e.g., middle cerebral artery or terminal internal carotid artery bifurcation, tip of the basilar trunk). Sidewall aneurysms are rare and if present usually arise at a turn or curve in the artery and point in the direction that the blood would have flowed if the curve were not present. At both the branch points and the curves, local alterations in intravascular hemodynamics are present that exert high shear stress forces on those regions that receive the greatest force of the pulse wave. Therefore, the aneurysm dome typically points in the direction of the maximal hemodynamic thrust in the pre-aneurysmal segment of the parent artery. Aneurysms that are encountered on a straight, nonbranching segment of an intracranial artery should raise the suspicion of a dissecting process because saccular aneurysms are infrequently encountered at these sites. More than 90% of all saccular intracranial aneurysms are located at one of the following five sites: (1) internal carotid artery at the origin of the posterior communicating artery; (2) junction of the anterior cerebral and anterior communicating arteries; (3) proximal bifurcation of the middle cerebral artery; (4) terminal bifurcation of the basilar artery into the posterior cerebral arteries; and (5) terminal bifurcation of the carotid artery into the anterior cerebral and middle cerebral arteries. In the anterior circulation, other common sites of aneurysms are the origins of the ophthalmic, superior hypophyseal, and anterior choroidal arteries. In the posterior circulation, the origin of the posterior inferior cerebellar, anterior inferior cerebellar, and superior cerebellar arteries and the junction of the basilar and vertebral arteries are likely sites of aneurysm formation. CT/CTA MRI/MRA CONSERVATIVE OR MEDICAL MANAGEMENT Published Literature on Treatment Options Given the high risk for rebleeding with its associated mortality, there is no question that acutely ruptured aneurysms should be treated. Concerning the timing of treatment, in most centers an acute treatment is advocated. Even with an early and successful intervention, SAH is a devastating disease, and the risk for a poor outcome is high. Factors associated with a poor outcome are poor clinical status on admission, a large SAH, increasing age, posterior circulation location of the aneurysm, and preexisting medical conditions. Neurologic complications due to hydrocephalus and vasospasm and systemic complications with infections, cardiac problems (arryhthmia, myocardial infarction), or pulmonary edema may all lead to a poor outcome. Basilar apex aneurysms arise where the posterior cerebral arteries branch off from the tip of the basilar artery. At the aneurysm site, the blood flow changes from vertical to nearly horizontal, so these aneurysms project upward in the direction of the long axis of the basilar artery. The majority of patients with basilar tip aneurysms display a relatively short basilar artery (caudal fusion disposition), with the tip of the basilar artery near the caudal end of the interpeduncular fossa. The neck of the basilar aneurysm is preferentially located at the caudal part of the fusion. The largest and most important perforators to arise from the basilar tip are the posterior thalamoperforating arteries (retromammillary arteries). These originate from the basilar tip and P1 segment, enter the brain through the posterior perforated substance in the interpeduncular fossa medial to the cerebral peduncles, and ascend through the midbrain to the thalamus. On occasion, a single P1 perforator may supply both thalami. Typically, the caudal portions of P1 supply their own sides, whereas the cranial segments of P1 may supply bilateral territories. In patients with caudal fusions of the basilar artery, the posterior communicating artery flows caudally and is likely to supply diencephalic-mesencephalic territories. In patients with cranial fusion of the basilar artery, there is likely to be a basilar contribution to the supply of this area. The risks from occlusion of these vital perforating vessels include visual loss, weakness, memory deficits, autonomic and endocrine imbalance, abnormal movements, diplopia, and depression of consciousness. Given the risk for surgical perforator occlusion, especially in larger aneurysms, endovascular approaches have been widely adopted to treat basilar apex aneurysms. The aim of endovascular treatment with platinum coils is to permanently occlude the aneurysm lumen based on the following assumptions: Immediately after the coils are deployed in the aneurysm, thrombocytes adhere to the coil loops because of changes in hemodynamics and flow velocity followed by fibrous organization of the primary thrombus over the course of the following days. According to the theory, mechanical stability is further increased over the next weeks by collagenous extracellular matrix and granulation tissue between the coil loops that may then create a foundation for neoendothelial sprouting from healthy vessel walls. Although these theoretical considerations seem appealing, experimental results are not always accordant; the newly developed thrombus, which mainly consists of erythrocytes captured within thin fibrin strings, may not be able to withstand the arterial blood pressure and the presence of fibrinolytic agents in the circulating blood. Moreover, physiologic thrombus retraction, the physical effects of the so-called water hammer effect on the coil material, and the possible presence of old thrombus within the aneurysm dome into which coil loops may be shifted, can lead to a delayed aneurysm regrowth. Despite the potential of delayed recanalization of the aneurysm, the rebleeding rates of sufficiently coiled aneurysms are low, and endovascular treatment of cerebral aneurysms is an established option in the management of ruptured aneurysms. PEARLS AND PITFALLS__________________________________________________ Bederson JB, Connolly ES Jr, Batjer HH, et al; American Heart Association. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 2009;40(3):994–1025 Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med 2006;355(9):928–939 Guglielmi G, Viñuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg 1991;75(1):8–14 Guglielmi G, Viñuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique, and experimental results. J Neurosurg 1991;75(1):1–7 Heros RC. Intracranial aneurysms. A review. Minn Med 1990;73(10):27–32 Pakarinen S. Incidence, aetiology, and prognosis of primary subarachnoid haemorrhage. A study based on 589 cases diagnosed in a defined urban population during a defined period. Acta Neurol Scand 1967;43(Suppl 29):29, 1–28 Peluso JP, van Rooij WJ, Sluzewski M, Beute GN. Coiling of basilar tip aneurysms: results in 154 consecutive patients with emphasis on recurrent haemorrhage and retreatment during mid- and long-term follow-up. J Neurol Neurosurg Psychiatry 2008;79(6):706–711 Sellar R, Molyneux A. ISAT: The International Subarachnoid Aneurysm Trial. Lessons and update. Interventional Neuroradiology 2008;14:50–51 van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369 (9558):306–318 A 51-year-old man has an acute onset of headache without loss of consciousness and without focal neurologic deficits. On arrival in the emergency department, he exhibits photophobia and nuchal rigidity. Emergency CT demonstrates a diffuse symmetric subarachnoid hemorrhage (SAH) and no evidence of hydrocephalus. CTA reveals a multilobulated anterior communicating artery aneurysm. Angiography is performed. Fig. 2.1 DSA. Right ICA angiogram in (A) AP and (B) lateral views and (C) 3D reconstruction reveals a lobulated outpouching at the anterior communicating artery pointing anteroinferiorly, representing a ruptured aneurysm. DSA After injection into the right internal carotid artery (ICA), the anterior communicating artery aneurysm was identified pointing anteriorly and inferiorly with at least three lobules, each measuring ~4 mm. The neck of the aneurysm measured 3.8 mm. A rather acute angle between the ICA termination and A1 was present (Fig. 2.1) Diagnosis Ruptured aneurysm of the anterior communicating artery Treatment The 6F multipurpose catheter was advanced into the right distal ICA. Over a J-shaped micro-guidewire, the acutely angulated A1 segment was catheterized by retracting the J-shaped guidewire from the M1 segment to hook up into the A1 segment and then slowly advancing both the microcatheter and the guidewire. To navigate the microcatheter around the acute curve, the guidewire was subsequently placed into the right distal A2 segment. Once the microcatheter was within the A1 segment, the guidewire was retracted and navigated into the aneurysm. The slack was removed from the system, and the catheter was slowly navigated into the distal lobulated part of the aneurysm. The first two coils were aimed at framing the distal part of the aneurysm. This part was then filled with DeltaPaq coils, which allowed good filling of the distal part of the aneurysm. The catheter was then gently retracted, and the second part of the aneurysm was coiled. Given its rounder shape, a MicruSphere coil was chosen for framing, followed by DeltaPaq coils for subsequent filling. The final result demonstrated complete occlusion of the aneurysm and no missing branches (Fig. 2.2) The patient had an uneventful recovery. For ruptured aneurysms, two major treatment options are available: neurosurgical clip ligation and endovascular coil treatment. Following its first clinical use in 1990, coil embolization was primarily reserved for those aneurysms that were difficult to access surgically and for patients with poor clinical grades. Since publication of the results of the International Subarachnoid Aneurysm Trial (ISAT), however, the endovascular treatment of cerebral aneurysms has gained more acceptance and is now regarded in most centers as the technique of choice for the treatment of ruptured aneurysms. The ISAT demonstrated that patients with aneurysms rated by both the neurosurgeon and the interventional neuroradiologist as possible candidates for their respective therapies had a better outcome concerning morbidity, dependency, and mortality when they underwent endovascular treatment. Approximately 10,000 patients with acute SAH were screened; of those, slightly more than 20% (n = 2143) were included in the study as eligible for both treatment options and were randomized to either surgery or coiling. The rate of death or dependency was 23.7% in the endovascular group and 30.6% in the surgical group at 1 year, resulting in relative and absolute risk reductions for death or dependency after endovascular treatment of 22.6% (95% CI [confidence interval], 8.9–34.2%) and 6.9% (95% CI, 2.5–11.3%), respectively. The study concluded that outcome in terms of survival free of disability at 1 year was better with endovascular options. The recently published longterm results (mean follow-up, 9 years; range, 6–14 years) confirm the positive trend for endovascular treatment options, with a significantly decreased risk for death at 5 years in the endovascular group (relative risk, 0.77; 95% CI, 0.61–0.98; p = 0.03). However, the proportions of survivors at 5 years who were independent did not differ between the two groups: endovascular 83% (626 of 755) and neurosurgical 82% (584 of 713). The risk for rebleeding was higher in the endovascular group (11 rebleeds vs. 2); however, it was still very low, with a cumulative risk for rebleeding after 6 years of ~1%. Fig. 2.2 Right ICA angiogram in the working projection following embolization of (A) the distal and (B) the proximal compartments. No filling of the aneurysm sac with contrast is seen after the coiling. Noninvasive and Invasive Imaging Workup SURGICAL TREATMENT See also Cases 6, 8, 9, and 10. Published Literature on Treatment Options The percentage of patients harboring a residual neck or body remnant of the aneurysm after coil treatment ranges between 5 and 20%, and 15 to 35% of patients will in time demonstrate recanalization of the aneurysm. Consequently, the risk for rebleeding is higher after coiling than after clipping. As late rebleeding occurs more often in patients treated by coil embolization (0.152%/y in ISAT, up to 0.3%/y in the literature vs. 0.03% for surgery), the question arises whether in younger patients with a longer life expectancy the superiority of the initial post-interventional results may be offset by the rate of late rebleeding. In addition, the ISAT subgroup analysis of patients younger than 40 years demonstrated that the difference between poor outcome rates of coiled versus clipped aneurysms was very small in this age group. This led to the conclusion in a recently published study by Mitchell et al that in the younger age group, clip ligation may be favored over coil placement. This notion is further supported by experimental results that demonstrated stable aneurysm occlusion, with neoendothelium covering the former aneurysm neck in clipped aneurysms, whereas in coiled aneurysms no stable occlusion was visualized. However, over the past years, endovascular methods have improved considerably, with new coils and device-assisted coiling, whereas there has been very little change in neurosurgical techniques. It is therefore likely that the rate of recurrent SAH will decrease while the safety of the endovascular treatments will increase. This was confirmed by Schaafsma et al in a study of recurrent SAH within the first 8 years after treatment in 283 coiled patients with a total follow-up of 1778 patient-years; the cumulative incidence was 0.4% (95% CI, –0.4 to 1.2) after coiling versus 2.6% (95% CI, 1.2–4.0) after clipping. The rates of rebleeding after adequate coiling of aneurysms are therefore equal to those after clipping. The results of ISAT, including the long-term results, indicate the superiority of endovascular treatment options for those aneurysms deemed suitable for both surgery and endovascular approaches. The results can therefore not be generalized to all ruptured aneurysms and all neurosurgical/endovascular teams. Instead, the results of ISAT must be extrapolated to individual patients after all the different factors that may play a role in the treatment decision have been considered, including the etiology of the aneurysm, the age and clinical status of the patient, the respective neurosurgical and endovascular experience and skills available, the anatomy and location of the aneurysm with regard to feasibility of the endovascular or surgical approach, and the equipment and material available and the comfort with which the treating team may use them. Finally, factors such as the health care environment and the patient or family’s wishes may play a role in the treatment decision. PEARLS AND PITFALLS__________________________________________________ Holmin S, Krings T, Ozanne A, et al. Intradural saccular aneurysms treated by Guglielmi detachable bare coils at a single institution between 1993 and 2005: clinical long-term follow-up for a total of 1810 patient-years in relation to morphological treatment results. Stroke 2008;39(8):2288–2297 Komotar RJ, Hahn DK, Kim GH, et al. Efficacy of lamina terminalis fenestration in reducing shunt-dependent hydrocephalus following aneurysmal subarachnoid hemorrhage: a systematic review. Clinical article. J Neurosurg 2009;111(1):147–154 Krings T, Busch C, Sellhaus B, et al. Long-term histological and scanning electron microscopy results of endovascular and operative treatments of experimentally induced aneurysms in the rabbit. Neurosurgery 2006;594):911–923, discussion 923–924 Mitchell P, Kerr R, Mendelow AD, Molyneux A. Could late rebleeding overturn the superiority of cranial aneurysm coil embolization over clip ligation seen in the International Subarachnoid Aneurysm Trial? J Neurosurg 2008;108(3):437–442 Molyneux AJ, Kerr RS, Birks J, et al; ISAT Collaborators. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT):long-term follow-up. Lancet Neurol 2009;8(5):427–433 Molyneux A, Kerr R, Stratton I, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. I nternational Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360(9342):1267–1274 Schaafsma JD, Sprengers ME, van Rooij WJ, et al. Long-term recurrent subarachnoid hemorrhage after adequate coiling versus clipping of ruptured intracranial aneurysms. Stroke 2009;40(5):1758–1763 van der Schaaf I, Algra A, Wermer M, et al. Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2005;(4):CD003085 Wermer MJ, Greebe P, Algra A, Rinkel GJ. Incidence of recurrent subarachnoid hemorrhage after clipping for ruptured intracranial aneurysms. Stroke 2005;36(11):2394–2399 Willinsky RA, Peltz J, da Costa L, Agid R, Farb RI, terBrugge KG. Clinical and angiographic follow-up of ruptured intracranial aneurysms treated with endovascular embolization. AJNR Am J Neuroradiol 2009;30(5):1035–1040 A 44-year-old woman with a long-standing history of migraines has what she describes as atypically severe headaches 4 days before admission. Because the headaches do not vanish and have increased slightly over time, she seeks the advice of her family physician, who orders CT. Fig. 3.1 (A) Unenhanced axial CT shows minimal symmetric hyperdensity along the suprasellar and MCA cisterns bilaterally. (B) Left ICA angiogram in AP view and (C) right ICA angiogram in lateral view reveal aneurysms at the left carotid termination and right posterior communicating artery origin. CT Unenhanced CT demonstrated a small amount of subarachnoidal blood with a symmetric distribution (Fig. 3.1A) There was mild enlargement of the ventricles. DSA After injection into the left internal carotid artery (ICA), a round carotid termination aneurysm was found, and injection into the right ICA demonstrated a posterior communicating artery aneurysm (Fig. 3.1 B,C) Diagnosis Multiple aneurysms Because it could not be decided which aneurysm was ruptured and because both aneurysms were not deemed accessible via the same craniotomy, embolization with occlusion of both aneurysms was performed in the same setting. The 6F multipurpose catheter was placed in the left ICA, and 3D angiography was performed to obtain the working projection. Catheterization and subsequent coiling of the left ICA termination aneurysm was performed under roadmap conditions in the working projection after framing with a complex (GDC 10 360-degree) coil and subsequent filling with soft helical coils. Subsequently, the same procedure was repeated for the right side with uneventful coiling of the right posterior communicating artery aneurysm(Fig. 3.2) The procedure was performed under heparin. The patient awoke from anesthesia without neurologic deficits and had an uneventful recovery. She will be followed with noninvasive imaging to evaluate the stability of the coiled aneurysms and to screen for possible de novo aneurysms. The incidence of multiple intracranial aneurysms appears to be extremely variable, depending in part on the patient population (whether the series included surgical, radiologic, or autopsy findings and whether the aneurysms were ruptured or unruptured). The prevalence of multiple intracranial aneurysms varies between 25 and 31% in autopsy series and between 12 and 26% in larger clinical series. Female patients account for 60 to 81% of those with multiple aneurysms. The ICA and middle cerebral artery (MCA) are most prone to the formation of multiple aneurysms. A special subgroup of multiple aneurysms are the “mirror-like” or “twin” aneurysms at symmetric sites on each side. Twin aneurysms occur in ~5 to 10% of all patients with aneurysms, but in as many as 36% of all patients with multiple aneurysms. They are found in all intracranial vessels but are most common in the MCA. They exhibit a familial association, and they tend to rupture earlier in life than “classic” aneurysms. If multiple aneurysms are found in a patient presenting with a subarachnoid hemorrhage (SAH), the question of which one of the aneurysms has ruptured must be raised. Imaging can in most instances help to define the culprit aneurysm, which can then be targeted for priority treatment. CT/MRI Published Literature on Treatment Options Surgery has been recommended for patients with multiple aneurysms because of the ability to treatthe diff erent aneurysms via the same surgical exposure and in the same surgical session. It has been estimated that between 50 and 70% of patients with multiple aneurysms can have all their aneurysms treated via a single craniotomy. Because multifocality may indicate an underlying vessel wall weakness with a subsequently higher risk for aneurysm regrowth over time, a more definitive treatment, msuch as clipping, may also speak in favor of surgery. However, for patients in whom multiple craniotomies would be necessary to treat all the aneurysms, the benefits of surgery are less obvious, and one may instead argue that an endovascular approach is much less invasive as long as the multiple aneurysms can be treated within a single endovascular setting, as was done in our case. In our practice, we first determine which is the ruptured aneurysm by means of imaging data, as outlined above. The treatment decision is then based on neurosurgical versus endovascular accessibility and predicted outcome, with the International Subarachnoid Aneurysm Trial (ISAT) data taken into account. It is only secondarily that the argument with respect to multiplicity is taken into account based on the considerations described in the first paragraph of this section. If we are unsure about mwhich aneurysm has ruptured, all aneurysms are treated at the same time. Previous aneurysmal SAH is considered a major risk factor for subsequent hemorrhage from another, yet unruptured aneurysm. Therefore, treatment of these aneurysms is recommended. In ISAT, of 24 observed rebleeds, 11 occurred from another aneurysm, four of which had been present at the initial angiogram and the remainder of which presented as de novo aneurysms. In a cohort of more than 600 patients treated with clipping, 129 aneurysms were detected after a mean interval of 8 years; 20% of these were at the site of previous rupture, 80% were remote from it. Thirty percent of them were truly de novo aneurysms, the others having already been there at presentation. Growth was noted in 25% of the followed aneurysms. This underlines in our opinion the need and benefit of continuous screening of patients with SAH to detect the development of additional aneurysms. In patients with multiple aneurysms, the physician should actively seek an underlying disease such as polycystic kidney disease, connective tissue diseases (fibromuscular dysplasia, Marfan syndrome, Ehlers-Danlos type 4 syndrome), neurofibromatosis, and familial occurrence with potential genetic influences, and also rarer diseases such as cardiac myxoma. Patients must be discouraged from smoking because smoking is a major risk factor for rebleeding from a secondary aneurysm. PEARLS AND PITFALLS__________________________________________________ Baccin CE, Krings T, Alvarez H, Ozanne A, Lasjaunias P. Multiple mirror-like intracranial aneurysms. Report of a case and review of the literature. Acta Neurochir (Wien) 2006;148(10):1091–1095, discussion 1095 Imhof HG, Yonekawa Y. Management of ruptured aneurysms combined with coexisting aneurysms. Acta Neurochir Suppl (Wien) 2005;94:93–96 Porter PJ, Mazighi M, Rodesch G, et al. Endovascular and surgical management of multiple intradural aneurysms: review of 122 patients managed between 1993 and 1999. Interventional Neuroradiology 2001;7(4):291–302 Ujiie H, Tamano Y, Sasaki K, Hori T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm “Neurosurgery 2001;48(3):495–502, discussion 502–503 Wermer MJ, van der Schaaf IC, Velthuis BK, Algra A, Buskens E, Rinkel GJ; ASTRA Study Group. Follow-up screening after subarachnoid haemorrhage: frequency and determinants of new aneurysms and enlargement of existing aneurysms. Brain 2005;128(Pt 10):2421–2429 A 20-year-old woman with a positive family history of subarachnoid hemorrhage (SAH) is screened for aneurysms. (The patient’s mother and the mother’s sister both had aneurysmal SAH.) MRA shows an internal carotid artery (ICA) termination aneurysm, and the patient is referred to us for further management. The patient is a heavy smoker. Fig. 4.1 DSA. Left ICA angiogram in (A) AP view and (B) 3D transparent volume rendering shows a left carotid termination aneurysm. DSA After injection into the left ICA, a carotid termination aneurysm was seen with a maximum diameter of 6 mm, a small neck, and no daughter aneurysms (Fig. 4.1) The remainder of the examination was unremarkable. Diagnosis Unruptured aneurysm of the ICA termination Although the International Study on Unruptured Aneurysms (ISUIA) reported a 5-year risk for the rupture of anterior circulation aneurysms less than 7 mm in size to be close to 0 and the treatment-related risk to be ~10%, treatment was decided upon in this case, given the expected lower risk of treatment in a young patient with a medium-size aneurysm having a small neck and the expected higher natural history risk in a patient with (1) a positive family history, (2) female gender, (3) a long life expectancy, and (4) a history of smoking. A 6F multipurpose catheter was introduced into the left ICA, and 3000 units of heparin were given. Following a roadmap in the working projection, the microcatheter was advanced over the micro-guidewire and placed close to the entrance of the aneurysm, from which deployment of the first coil was started. Once this coil was deployed, the catheter was slightly advanced into the aneurysm, and subsequent coiling with smaller and softer coils was performed until a dense coil mesh was obtained. Control series via the guiding catheter demonstrated complete occlusion of the aneurysm (Fig. 4.2) The micro-guidewire was advanced, straightening the catheter, and the microcatheter was slowly withdrawn from the aneurysm, with care taken to prevent any coil loops from exiting the aneurysm. The patient awoke without any neurologic deficits. Follow-up control after 1 year demonstrated stable results and no de novo formation of aneurysms. Fig. 4.2 DSA. Left ICA angiograms in the working projection (A) before and (B) after coiling. (C) Final control angiogram of the left ICA in AP view shows no residual filling of the aneurysm sac. Depending on the study cited, the prevalence of arterial aneurysms is between 2 and 5% of the general population, approximately 10 times higher than the frequency of arteriovenous malformations in the same group. In a recent meta-analysis, a prevalence of intracranial aneurysms in adults of 2.3% was found, with an increasing prevalence in elderly patients and a slight predominance in females. A positive family history and the presence of polycystic kidney disease were associated with a higher rate of intracranial aneurysms. Given the high morbidity and mortality rates associated with aneurysms, the treatment of incidental aneurysms has to be based on a comparison of the risks of their natural history versus the risks of treatment. The risk for rupture of intracranial aneurysms was evaluated in a recent meta-analysis by Wermer et al. They found that the risk for rupture was increased in female patients, in those with a history of smoking, in elderly patients, and in patients of Japanese or Finnish descent. Larger aneurysms, posterior circulation aneurysms, and aneurysms in patients who had other, previously ruptured aneurysms were also associated with a higher risk for hemorrhage. This meta-analysis found the overall annual risk for hemorrhage of an unruptured aneurysm to be 0.7%. In the WHO MONICA study of stroke by Ingall et al, a total of 3368 SAH events recorded during 35.9 million person-years of observation in 11 populations in Europe and China comprising 25- to 64-year-old men and women resulted in SAH rates of 7 to 21 per 100,000 persons per year. Given the rate of aneurysm occurrence of ~2%, a risk for rupture of unruptured aneurysms of 0.3 to 1% per year can therefore be expected. These values (derived from a meta-analysis of 19 studies including ISUIA and a validated prospective population-based study) are in striking contradiction to those of ISUIA 2 and ISUIA 3. ISUIA prospectively investigated the natural history of unruptured aneurysms and evaluated the risks of surgical and endovascular treatment in 4060 patients, 1692 of whom received no treatment. Table 4.1 demonstrates the cumulative risks for bleeding in 5 years stratified according to aneurysm location (anterior circulation [excluding the posterior communicating artery] versus posterior circulation), aneurysm size, and history of previously bled aneurysm (group 2) versus no previously ruptured aneurysm (group 1). The discrepancy between these findings and our clinical experience in daily practice (i.e., most ruptured aneurysms are anterior circulation aneurysms < 7 mm in size) has been the subject of considerable discussion. Likewise, the procedural risks reported in this study (a surgical therapeutic risk of slightly > 10% and an endovascular risk of ~9%) are in striking contrast to those reported in other, larger studies on treatment-related risks, which are in the range of 4%. Although it is beyond the scope of this book, which is a case-based compilation of endovascular procedures, to discuss in detail the potential shortcomings of ISUIA, the following comments can be made: The hemorrhagic risk in the anterior circulation, despite being low, is implausibly low at 0; likewise, the procedural risk of 10% is implausibly high. The study does not take into consideration individual features of the aneurysm (e.g., aneurysm morphology [aspect ratio, blebs, irregular shape], aneurysm growth over time, intra-aneurysmal flow dynamics or associated vessel wall disease, aneurysm etiology, associated calcifications, multiplicity of aneurysms, associated arterial variations), nor does it account for individual patient characteristics (female vs. male gender, family history of aneurysms or SAH, history of smoking or hypertension, ethnic descent). Finally, it does not take into account the experience of the local treatment team that the neurointerventionalists and neurosurgeons must quote to the patient during the process of informed consent. Therefore, a strict adherence to the numbers in this study without a consideration of the individual patient, the individual aneurysm, and the experience of the local treating team is in our opinion not correct. CT/CTA MRI/MRA CONSERVATIVE OR MEDICAL MANAGEMENT Endovascular treatment options for small unruptured aneurysms are coil embolization (with or without device assistance; e.g., stent, balloon). In small-necked aneurysms, like the one in this case, unassisted endovascular coiling is in most instances possible. For unruptured aneurysms, we tend to use the slightly stiffer 18 coils. Published Literature on Treatment Options Based on the above-mentioned considerations regarding the shortcomings of ISUIA and based on other studies of the natural history risk of unruptured aneurysms, we recommend the treatment of posterior circulation unruptured intracranial aneurysms (including those of the posterior communicating artery) larger than 3 mm and the treatment of anterior circulation aneurysms larger than 7 mm. The treatment of anterior circulation unruptured aneurysms smaller than 7 mm should be individualized based on the age and family history of the patient, previous hemorrhage, and the aneurysm shape and growth. If conservative management is adopted, follow-up noninvasive imaging is performed in our center (preferably with unenhanced TOF MRA at 3 T) to rule out aneurysm growth over time. If invasive management is proposed, then as the next step the treatment modality has to be determined. Again, this has to be individualized based on the age of the patient, the location and shape of the aneurysm, the treatment team’s capability (including its morbidity and mortality record), and the patient’s preference. In ISUIA, there was a slightly better outcome in patients treated by endovascular means. However, this has to be weighed against (1) the higher risk for residual aneurysms after coiling (5–20% in the literature following coiling, 0–3% after clipping), (2) the higher risk for aneurysm recanalization after coiling (15–35% vs. 0–5%), and consequently the higher re-treatment rates following coiling (5–13%). In addition to these considerations, decisions about what therapy to choose depend on the location of the aneurysm (with posterior circulation aneurysms more likely candidates for endovascular treatments), its morphology (incorporation of vessels at the aneurysm neck favors surgical techniques, whereas perforators surrounding the neck are more easily treated by endovascular options), and its size (very small and very large aneurysms are more likely to be treated surgically). If calcifications are present at the aneurysm neck, surgery may be more dangerous. On the other hand, in patients with kidney disease, the contrast material and the necessity of follow-up studies will likely favor surgical therapy. In patients with significant atherosclerosis and significant tortuosity and ectasia of the vasculature, endovascular access may be difficult and dangerous. PEARLS AND PITFALLS__________________________________________________ Clarke M. Systematic review of reviews of risk factors for intracranial aneurysms. Neuroradiology 2008;50(8):653–664 Ingall T, Asplund K, Mahonen M, Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke 2000;31(5):1054–1061 Pierot L, Spelle L, Vitry F; ATENA Investigators. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke 2008;39(9):2497–2504 Magnetic Resonance Angiography in Relatives of Patients with Subarachnoid Hemorrhage Study Group. Risks and benefits of screening for intracranial aneurysms in first-degree relatives of patients with sporadic subarachnoid hemorrhage. N Engl J Med 1999;341(18):1344–1350 Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 1998;29(1):251–256 Schievink WI, Schaid DJ, Michels VV, Piepgras DG. Familial aneurysmal subarachnoid hemorrhage: a community-based study. J Neurosurg 1995;83(3):426–429 Standhardt H, Boecher-Schwarz H, Gruber A, Benesch T, Knosp E, Bavinzski G. Endovascular treatment of unruptured intracranial aneurysms with Guglielmi detachable coils: short- and long-term results of a single-centre series. Stroke 2008;39(3):899–904 van Rooij WJ, Sluzewski M. Procedural morbidity and mortality of elective coil treatment of unruptured intracranial aneurysms. AJNR Am J Neuroradiol 2006;27(8):1678–1680 Wermer MJ, Rinkel GJ, van Gijn J. Repeated screening for intracranial aneurysms in familial subarach-noid hemorrhage. Stroke 2003;34(12):2788–2791 Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke 2007;38(4):1404–1410 Wiebers DO, Whisnant JP, Huston JIII, et al; International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362(9378):103–110 A 57-year-old woman is found unconscious by her daughter. On arrival in the emergency department, she has a Glasgow Coma Scale score of 5. Emergency CT demonstrates subarachnoid hemorrhage with a significant amount of intraventricular blood. Following bilateral external ventricular drainages, the patient localizes to pain stimulation and treatment is contemplated. Fig. 5.1 (A) Unenhanced axial CT shows diffuse subarachnoid hemorrhage bilaterally and focal clot within the interhemispheric fissure. Intraventricular hemorrhage in the lateral ventricles is also observed bilaterally. (B) 3D CTA reveals a large lobulated aneurysm of the anterior communicating artery. CT/CTA CT demonstrated bilateral symmetric blood, intraventricular hemorrhage, and blood in the inter-hemispheric fissure. CTA demonstrated as the source of bleeding a lobulated 4 × 3-mm anterior communicating artery aneurysm with a 3.5–mm broad neck (Fig. 5.1). Wide-necked ruptured aneurysm of the anterior communicating artery Treatment The 6F multipurpose catheter was placed in the left internal carotid artery (ICA). An attempt was made to navigate the balloon from the left ICA across the anterior communicating artery and the aneurysm neck into the right A2 segment; however, the guidewire could not be positioned into the A2 segment. Subsequently, the guiding catheter was navigated into the right ICA, from which balloon placement over the neck into the left A2 segment proved possible. Given the caliber of the A1 segment, we decided to introduce the microcatheter from the left side, and a second guiding catheter was introduced. The microcatheter could be introduced into the aneurysm, where the first coil was subsequently deployed with the balloon inflated to prevent coil protrusion into the parent artery. Deflation of the balloon verified stability of the coil, which was then detached (Fig. 5.2). The same procedure was repeated for the other coils, resulting in complete occlusion of the aneurysm with no evidence of thromboembolic complications (Fig. 5.3). At the end of the procedure, given the rather broad neck of the aneurysm, heparin was not reversed. Fig. 5.2 (A) 3D angiography of the right ICA in oblique AP view reveals the wide neck of the anterior communicating artery aneurysm. (B)

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree