PART XSpine Percutaneous Interventions
A 56-year-old man presents with paresthesia in the left lower limb in the L5 distribution. He was successfully treated for prostate carcinoma several years ago. CT is requested to evaluate the possible compression of neural elements in the lumbar spine.
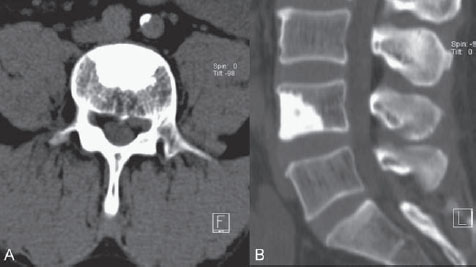
Fig. 66.1 (A,B) CT of the lumbar spine.
CT
CT of the lumbar spine demonstrated a left-sided paramedian disk herniation at the L4-L5 level with compression of the left L5 nerve root. As an incidental finding, a large osteodense lesion with irregular spicular margins was noted in the L4 vertebral body (Fig. 66.1). Given the absence of this lesion on CT scans 4 years previously, together with the patient’s history, an osteoblastic malignant lesion was suspected. Comparable lesions in other locations were not detected on extensive CT imaging of the thorax and abdomen or on bone scintigraphy. Therefore, a percutaneous biopsy of the lesion in the L4 vertebral body was planned.
EQUIPMENT
- Lidocaine (Linisol 2%; B Braun, Melsungen, Germany)
- Cutter (surgical blade)
- An 11-gauge, 10-cm M1M Cook bone biopsy needle with a beveled edge
- Kocher clamp (used to hold the biopsy needle)
- Orthopedic hammer
- A 20-mL syringe
- Closed recipient partially filled with 10% formalin
DESCRIPTION
After disinfection and local anesthesia, a 4- to 5-mm-wide incision was made in the skin, and the bone biopsy needle was introduced. With fluoroscopy, the bone biopsy needle was advanced toward the superolateral aspect of the right pedicle until bone contact was felt.
With the tip of the needle in contact with the bone, its position was determined on fluoroscopy to be just superior to the pedicle. Additional local anesthetic was injected through the biopsy needle to anesthetize the periosteum. Firm but controlled pressure, if necessary with rotation of the needle, was used to penetrate the cortical bone. (The needle tip should never pass beyond the inner margin of the pedicle on the AP view unless it has reached the posterior wall of the vertebral body on the lateral view.)
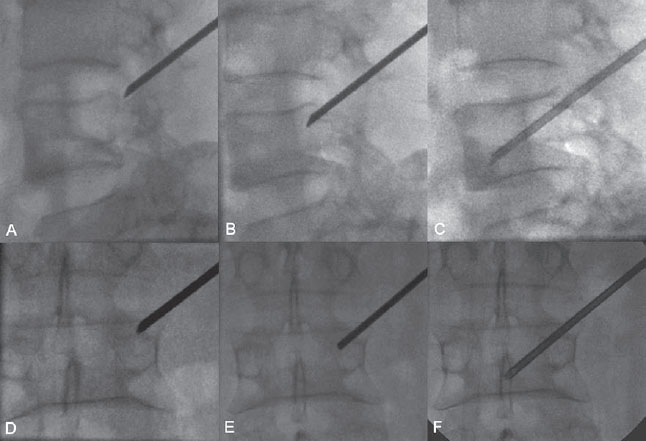
With lateral fluoroscopy, the needle tip position was determined to be superolateral within the vertebral body. The beveled edge was turned cranially and slightly laterally to help the needle tip slide caudally and medially toward the lesion(Fig. 66.2).
Fig. 66.2 (A–F) Fluoroscopy guidance in lateral and PA views with the use of a low dose of radiation.
In this patient, it was necessary to tap the needle with a small orthopedic hammer to penetrate the osteodense lesion. After the lesion had been entered for a distance of more than 5 mm, the biopsy needle was rotated under pressure and retracted while negative pressure was maintained with a 20mL syringe mounted on the hollow biopsy needle. (In the authors’ experience, this may help to keep the biopsy sample within the biopsy needle.) The inner needle was used to push the biopsy sample from the biopsy needle into the recipient. The biopsy sample was visually inspected; the transition zone from normal bone marrow (red color) to abnormal bone marrow (white color) was seen, and the size of the sample of abnormal bone marrow was considered sufficient for pathologic examination. No second biopsy sample was taken. Pathology revealed infiltration by an osteoblastic tumor that was a prostate cancer metastasis.
Vertebral biopsy is often indicated in cases of suspected neoplastic lesions or infectious spondylitis. Historically, open surgical biopsy of the spine was the only option for a definitive diagnosis. In recent decades, percutaneous, image-guided vertebral biopsy procedures have been developed and are routinely used; these procedures are faster and more cost-effective, with an overall lower risk for complications. Fluoroscopy and CT are most commonly used for image guidance. The reported accuracy of core biopsy ranges from 77 to 97%.
Direct communication between the radiologist and referring physician, with a review of previous imaging and laboratory examinations, is needed to establish an indication for percutaneous vertebral biopsy; the benefits of a definitive diagnosis of a suspected vertebral lesion by biopsy must outweigh the risks of the biopsy procedure.
PHYSICAL EXAMINATION/LABORATORY RESULTS
- Exclude skin lesions (e.g., infection, wound, hemangioma) at the expected skin entry site.
- Laboratory parameters to be determined prior to the procedure are as follows: hematocrit, hemoglobin, platelet count, prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), blood urea nitrogen (BUN), and creatinine.
- Interrogate the patient regarding allergies and the use of anticoagulants.
CT/MRI
- Find other, similar lesions at locations that are more easily reached (with a lower risk for complications) for biopsy.
- Evaluate the location and size of the lesion to determine the biopsy trajectory, size of the needle, and type of imaging guidance.
- Evaluate for any associated soft-tissue mass effect of the lesion; sometimes, such tissue can more easily be reached for biopsy.
- Evaluate for intraspinal and foraminal tumoral extension. Be aware that mass effect on the thecal sac or nerve roots may increase in case of hemorrhage during or after the procedure.
- Evaluate if the lesion is sufficiently visible on plain film.
- In case of bone destruction, evaluate if the vertebral outlines necessary for visual navigation of the biopsy needle are sufficiently visible or can be inferred adequately from adjacent vertebral structures. For example, enlargement and bone destruction may cause a pedicle to be invisible on plain film; however, a biopsy can be performed confidently in such a pedicle by using other landmarks of the vertebral body or adjacent vertebrae.
- Primary bone lesions and tumors
- Secondary bone tumors
- Infectious spondylitis/spondylodiscitis
SURGICAL PROCEDURE
- Open surgical biopsy may make it possible to obtain larger samples and multiple samples.
- Open surgical biopsy is preferred if the patient is undergoing spinal decompression or stabilization at the same time.
PERCUTANEOUS PROCEDURE
- In our practice, we most often use local anesthesia of the skin and periosteum and reassure the patient verbally. General anesthesia is rarely needed and should be reserved for surgical open biopsy.
- A large array of percutaneous biopsy needles and systems is available. Stylet-bearing 20- or 22-gauge needles can be used for aspiration biopsy. Trephine or beveled-tip 10- to 14-gauge needles are available for core biopsy.
- A core biopsy can be obtained with a coaxial or a tandem technique. In the tandem technique, the needle used for local anesthesia is directed toward the lesion and serves as a visual guide for placing the biopsy needle alongside it. In the coaxial technique, the biopsy needle is placed over the guiding needle (removable hub), and multiple needle passes for biopsy can be performed through the guiding cannula, which remains in place.
- Beveled-tip needles have two intrinsic advantages. Inside the pedicle, the beveled side is turned toward the medial margin of the pedicle to avoid cortical penetration and injury to structures within the spinal canal; inside the vertebral body, the bevel can be used to push against bone, and the needle tip may be redirected slightly away from the beveled side.
- Several approaches to the vertebral body are possible. At lumbar levels, a trans-pedicular or posterolateral approach is used. Thoracic vertebral bodies are entered via a trans-pedicular or trans-costovertebral approach. Cervical levels are most frequently biopsied via an anterolateral approach, with displacement of the carotid vessels.
- Hematoma and active bleeding: When a trans-pedicular approach is used, hematoma can be avoided by applying pressure to the soft tissues manually after the biopsy needle has been withdrawn. However, when a posterolateral approach is used, retroperitoneal bleeding may occur (which cannot be compressed manually).
- Vascular injury may be the result of direct puncture of arteries or veins, most notably the aorta and inferior vena cava adjacent to the vertebral bodies.
- Neural injury may result from direct puncture of spinal cord or nerves or from an indirect mechanism through vascular injury (anterior spinal artery).
- Pneumothorax may occur.
- Rib fracture: Forceful pressure on the patient’s back may cause a rib fracture (e.g., in a patient with osteoporosis).
- Infection may develop, and possibly intraspinal abscess or meningitis.
Published Literature on Treatment Options
Percutaneous spine biopsy has widely replaced open biopsy. The adequacy, accuracy, and complication rates of percutaneous spine biopsy increase with the inner diameter of the biopsy needles used. CT guidance and fluoroscopy guidance do not differ significantly in adequacy and accuracy of tissue sampling. The complication rate of 5.3% for fluoroscopy is higher than the rate of 3.3% for CT guidance. The type, level, and vertebral location of the lesion, together with the expertise of the physician, may determine the choice between fluoroscopy or CT guidance.
Percutaneous spine biopsy has a specific role in reaching the definitive diagnosis of suspected vertebral lesions; a combined clinical, radiologic, and pathologic approach to these lesions provides an excellent diagnostic yield.
PEARLS AND PITFALLS__________________________________________________
- Review the indication for biopsy with the referring physician, and review the patient’s chart (coagulation, allergy).
- Anesthetize the skin, and also the periosteum.
- A Kocher clamp is used to hold the needle during intermittent fluoroscopy, so that the hands of the performing physician are not exposed to radiation. Pulsed fluoroscopy, a low tube current, and a small field of view are key to minimizing the physician’s and patient’s exposure to radiation while providing sufficient image quality. Some physicians prefer to use leaded gloves.
- Always know the exact position of the needle tip by using both lateral and PA fluoroscopy
- If the lesions are too small or the vertebral landmarks are not easy to locate at fluoroscopy, use CT guidance.
Geremia G, Joglekar S. Percutaneous needle biopsy of the spine. Neuroimaging Clin N Am 2000;10(3):503–533
Nourbakhsh A, Grady JJ, Garges KJ. Percutaneous spine biopsy: a meta-analysis. J Bone Joint Surg Am 2008;90(8):1722–1725
Stoker DJ, Kissin CM. Percutaneous vertebral biopsy: a review of 135 cases. Clin Radiol 1985;36(6):569–577
Tehranzadeh J, Tao C, Browning CA. Percutaneous needle biopsy of the spine. Acta Radiol 2007;48(8):860–868
A 72-year-old woman living in a coastal city enjoyed her daily long walks on the beach. She has no previous medical history. One morning, she wakes up with a severely painful back, which leaves her immobilized in bed. Rest and pain medication are not helpful in alleviating the pain, and with great difficulty she manages to get to the local hospital a few days later. Laboratory findings are normal. Clinical examination and plain films of the lumbar spine are not considered suggestive of painful vertebral compression fracture (VCF). The patient is sent back home with a presumed diagnosis of fecal impaction but is bedridden with persistent pain. Her nephew, a nurse at the radiology department of our hospital, 100 miles inland, arranges to transfer the patient to our unit for MRI.
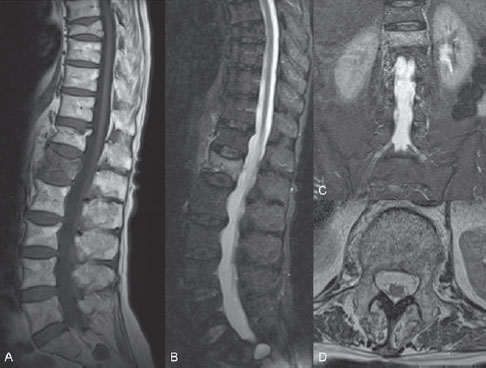
Fig. 67.1 (A–D) Sagittal T1-weighted and STIR MR images, coronal STIR image, and transverse T2-weighted MR image.
MRI
MRI of the lumbar spine revealed multiple VCFs. The VCFs at levels L3, T12, and T8 demonstrated normal bone marrow signal on STIR (short T1 inversion recovery) and T1-weighted images and were considered healed fractures. The VCF at level L1 showed diffusely increased signal on STIR images and decreased signal on T1-weighted images, strongly suggestive of an acute VCF. Coronal STIR images with a large field of view excluded the presence of acute VCFs at other levels and demonstrated no sacral insufficiency fracture. Transverse T2-weighted images excluded displacement of the posterior wall of the vertebral body and medullary compression. The latter images were useful to plan the trans-pedicular bone needle trajectory for vertebroplasty by demonstrating the size and direction of the pedicles (Fig. 67.1).
Acute VCF of L1
EQUIPMENT
- Kocher clamp
- Cutter
- Two 11-gauge, 10-cm Cook M1M bone needles with beveled tips
- Cement (liquid monomer and powder)
- One 20-mLJanet-type syringe
- Seven 1-mL syringes with reinforced plungers (Medallion)
- Steri-Strips
DESCRIPTION
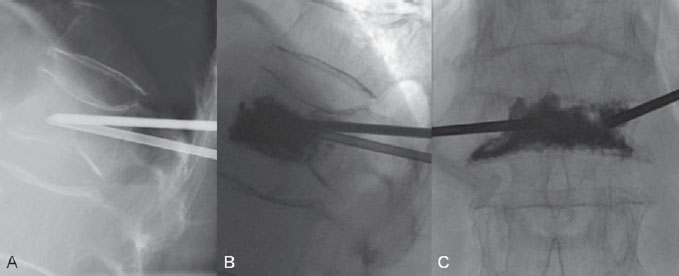
The patient underwent general anesthesia and was gently turned into a prone position on the table in the angiography suite. Supporting cushions at the upper thorax and pelvis helped to bring the spine into a hyperlordotic position (sometimes helpful for restoring height) and to prevent rib fractures. Sterile draping and sterile cleansing of the skin were done. On the left, a trans-pedicular approach toward vertebral body L1 was chosen. The skin entry site was determined with PA fluoroscopy, and a 5-mm-wide skin incision was performed. The skin entry site was 2 cm lateral to the pedicle, and the needle was pushed through the soft tissues toward the outer margin of the pedicle until bone contact was felt. With the tip of the needle between the mammillary process and accessory process of the pedicle and with the bevel turned laterally, the cortical bone could be penetrated by using short rotating movements. Once the needle tip was inside the pedicle, its position was checked on lateral fluoroscopy. On PA view, the needle was advanced deeper into the pedicle. Care was taken not to pass beyond the inner or lower margin of the pedicle to avoid intraspinal or nerve root damage; therefore, the bevel was turned medially or inferiorly if the needle tip approached the margins on the PA view. Once the needle tip was inside the vertebral body on the lateral view, the bevel could be turned laterally again to help slide the needle tip toward the midline of the vertebral body. On the right, an extrapedicular approach with a more craniocaudal trajectory was chosen, and therefore the skin entry site was 2 cm lateral and 2 cm superior to the pedicle. The needle was advanced in a craniocaudal direction through the soft tissues and aimed at the superolateral side of the pedicle until bone contact was felt. Cortical bone on the superior side of the pedicle base or on the postero-superior side of the vertebral body was penetrated with the bevel turned cranially. Once inside the trabecular bone of the vertebral body, the needle was advanced toward the midline of the vertebral body with the bevel directed cranially and/or laterally (Fig. 67.2).
After both needle tips were positioned in the anterior one-third of the vertebral body, cement was prepared. A 20-mL Janet-type syringe was filled with the liquid cement and used to fill the 1-mL syringes. The syringe filled first was used to intermittently test the liquidity of the cement. While the plunger is pushed gently, the liquid cement initially drips out of the syringe, and as the cement slowly becomes more viscous, the drip rate slows. When the cement curls like tooth paste at the tip of the syringe and does not drip any longer, it is ready to be injected.
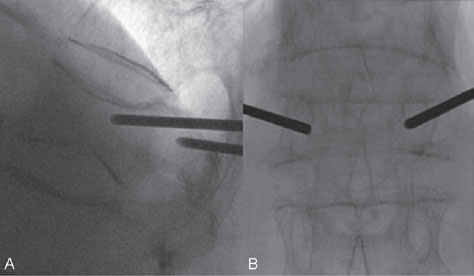
Cement was injected very slowly under continuous fluoroscopic control in the lateral view as careful attention was paid to the spread of cement inside the vertebral body and eventually to stop the cement injection in case of leakage outside the vertebral body. A trabecular spread of cement (honeycomb or spicular pattern) is indicative of the interdigitation of cement with trabecular bone (Fig. 67.3). After the inner needles were repositioned within the bone needles (pushing the cement out of the bone needles), the bone needles were retracted without causing a cement antenna in the soft tissues. The skin incisions were cleansed and closed with Steri-Strips. After 10 minutes of cement hardening, the patient was turned to a supine position and awakened. She had to remain in bed (supine position on a flat mattress) for 4 hours to allow the cement to harden completely. The patient was discharged the next day and returned within a week to her normal level of daily activity with strongly reduced pain scores. The patient has now resumed her long walks on the beach and invites her nephew to come along.
Fig. 67.3 (A–C) Lateral and PA views. With the needle tips in the anterior one-third of the vertebral body, cement is slowly injected with the use of continuous lateral fluoroscopy. Note the trabecular spread of cement.
Osteoporosis is a skeletal condition characterized by low bone mass and abnormal bony microarchitecture, which lead to an increased risk for fracture with minimal trauma. Aging, menopause, and other factors, such as alcohol and smoking, can result in bone loss and eventually low bone mass. Vertebral fractures account for almost half of all symptomatic osteoporotic fractures, and patients with these fractures have an increased mortality (up to 25–30%) compared with age-matched controls. In the Unites States, 700,000 to 1,200,000 new VCFs result from osteoporosis each year, with direct costs exceeding $15 billion. The risk for subsequent vertebral fracture is sevenfold to 10-fold higher in individuals who sustained a previous vertebral fracture, and the incidence of a new vertebral fracture within a year after a vertebral fracture approaches 20%.
Deramond and Galibert in France performed the first percutaneous vertebroplasty in 1984 when they injected bone cement into a C2 vertebral body weakened by an aggressive hemangioma. The extensive French experience with this technique was presented in 1993 at the second World Federation of Interventional and Therapeutic Neuroradiology Congress in Vancouver, BC, Canada, after which several North American teams became interested in the technique. In the United States, it began to be applied widely after investigators at the University of Virginia reported an 85 to 90% rate of significant pain relief in patients with osteoporotic compression fractures. During the following decade, percutaneous vertebroplasty developed into a routine clinical technique that is indicated for patients with pain resulting from osteoporotic VCFs that are not effectively treated by medical or conservative therapy. Additional indications were established for patients with painful destruction of vertebral bodies resulting from multiple myeloma, aggressive hemangioma, or metastasis.
PHYSICAL EXAMINATION AND LABORATORY ANALYSIS
- It is important to correlate the symptoms and location of pain with the findings on imaging (MRI); do not base a treatment decision on the imaging findings alone. Tapping the spinal processes and an axial compression test of the spine are useful to locate the source of back pain. Pain radiating into the lower limbs may be present but should not be the primary indication for cement augmentation
- Use pain scores (Visual Analogue Scale) and scores of daily life activity (e.g., Roland-Morris Disability Index) to evaluate patients before and after treatment.
- A routine blood analysis, including coagulation parameters, is necessary before the procedure
RADIOGRAPHY
- Strictly lateral and AP radiographs are used.
- Apply the Genant classification: semiquantitative visual grading of vertebral fractures regarding height loss (grade 0: normal height; grade 1 or mild fracture: 20–25% height loss; grade 2 or moderate fracture: 25–40% height loss; grade 3 or severe fracture: ≥40% height loss) and shape (wedge, biconcave, or crush fracture).
- CT can be useful to evaluate displacement of the posterior wall, cortical breaches, and the direction of trabecular fracture lines (predicting cement distribution or leakage).
- CT can be used to search for signs of pathologic fracture, such as osteolytic areas or soft-tissue and epidural mass.
MRI
- Use inversion recovery (STIR or TIR [turbo inversion recovery]) sequences to detect bone marrow edema; T2-weighted sequences with fat saturation may be used as an alternative. Confirm areas of bone marrow edema on T1-weighted images.
- Add a coronal STIR sequence with a large field of view to detect other recent VCFs or paravertebral rib fractures, and to exclude (insufficiency) fractures of the sacrum (sacral wings).
- Evaluate bone marrow edema to confirm acute fracture or incompletely healed fracture. Differentiate bone marrow edema due to fracture from Modic type 1 changes, tumor, inflammatory spondylitis, and infectious spondylodiscitis.
- Search for signs of pathologic fracture, tumoral bone marrow involvement, or soft-tissue mass.
- In case of posterior wall displacement, evaluate possible compression of the cord or the cauda equina.
- Detect bone marrow edema in posterior elements of the vertebrae, such as the lamina and spinous process, and soft-tissue edema in interspinous or supraspinous ligaments; these findings in association with a (wedge) fracture of the vertebral body may indicate an increased risk for delayed healing or further compression if conservative treatment is chosen.
- Detect bone marrow edema in the pedicles; if a fracture in the pedicles is present, one may leave a cement antenna within the pedicle at the end of the cement injection when retracting the working cannula that is positioned following a trans-pedicular trajectory.
- Assess the size and direction of the pedicles of the fractured vertebra.
BONE MINERAL DENSITOMETRY
- Bone mineral densitometry is useful to establish the diagnosis of (postmenopausal) osteoporosis and to predict fracture risk. In Caucasian American women older than 64 years of age, for each decrease of one standard deviation in bone mineral density at the hip (Z-score), the relative risk for hip fracture increases 2.6-fold.
- Dual-energy x-ray absorptiometry (DEXA) is a 2D projection technique, and osteoporotic change may sometimes be obscured in the spine. Impacted, fractured bone is more compact and therefore may appear denser, and degenerative changes with osteophyte formation and subchondral sclerosis may also result in increased density. Therefore, an additional measurement of bone mineral density at the femoral neck is useful (except for patients with bilateral hip prostheses).
NUCLEAR MEDICINE STUDIES
- Imaging is performed 3 hours after the intravenous injection of technetium Tc 99m methylene diphosphonate (555–925 MBq).
- Increased tracer uptake reflects osteoblastic activity in bone; bandlike, intense tracer accumulation is seen in the vertebral bodies in osteoporotic or traumatic VCFs.
- In elderly patients, imaging should be delayed for 72 hours after trauma (or the onset of pain) because osteoblastic bone repair may be slower or weaker in them than in younger individuals.
- Tracer accumulation in vertebral fractures is very intense during the first months, then gradually diminishes to nearly normal after 18 to 24 months; therefore, the bone scan is able to distinguish between (sub)acute and older collapses.
- Nuclear medicine studies easily detect additional VCFs and paravertebral rib fractures.
- VCF caused by weakened bone associated with primary or secondary bone tumors or osteomalacia
- Rib fracture (with or without VCF)
CONSERVATIVE OR MEDICAL MANAGEMENT
- Pain medication
- Relative bed rest
- External bracing
- Physiotherapy
- Peroral or intravenous biphosphonates
PERCUTANEOUS TREATMENT
- Percutaneous vertebroplasty
- Percutaneous kyphoplasty (performed with a balloon, a balloon and stent, or a radiofrequency technique)
SURGICAL TREATMENT
- Instrumented (posterior) vertebral fixation
- During instrument positioning: Damage to intraspinal or paraspinal neural or vascular structures may occur.
- During cement injection: If the cement is too liquid during injection, it will not displace the bone marrow. Instead, it will enter the vascular channels and leak outside the vertebral body into para-vertebral veins. This liquid cement may flow quickly toward the right atrium of the heart and form cement emboli in the pulmonary arteries. If a patent foramen ovale is present, cement emboli may even cause paradoxical infarctions. Cement leakage into vascular channels connecting to the central veins posteriorly in the vertebral body may cause cement to enter the anterior epidural venous plexus. Cement leakage in the paravertebral soft tissues is less important except when the upper side of the neural foramen is involved (injury to exiting nerve root by compression or thermal effect) or when the cement leaks into the spinal canal (compressing intraspinal neural structures). Cement leakage into the intervertebral disk space is clinically less important, but in some cases it may cause acute, painful Schmorl’s nodes or even endplate fractures of adjacent vertebrae.
- When the bone needles are retracted after cement injection, cement antennas in the posterior soft tissues should be avoided. Therefore, all cement should be pushed out of the bone needles into the vertebral body, and the bone needles should be retracted with the use of lateral fluoroscopy. If cement is left in the bone needle, one should wait until the cement has hardened before retracting the needle (wiggling the bone needle may help to break the cement cylinder at the transition between bone and soft tissue). If, nevertheless, a cement cylinder forms in the soft tissues, one may often be able to retrieve it with the bone needle under fluoroscopy.
- After the procedure, additional VCFs may occur at other (adjacent or nonadjacent) levels.
Published Literature on Treatment Options
Conventional open surgery for vertebral fractures is associated with risks resulting from open reduction and internal fixation and is therefore usually reserved for fractures that cause neurologic impairment. Surgical fixation in osteoporotic bone has been unsuccessful because of loosening of the intra-osseous screws.
Percutaneous vertebroplasty, a minimally invasive procedure, has been shown to have substantial benefits for patients with osteoporotic VCFs, in particular during the first month after fracture. These include considerable and often immediate reduction of pain, improved ability to perform the activities of daily living, decreased use of pain medication, and shorter hospital stay.
Recently, two randomized studies published in The New England Journal of Medicine have questioned the benefits of percutaneous vertebroplasty; the results of these studies suggested that percutaneous vertebroplasty is no more effective than placebo treatment. This contradicts the personal experience of many radiologists and surgeons, as well as the results of most of the studies previously published. A commentary of the North American Spine Society suggested that potential confounding factors (e.g., patient selection) might have caused the described neutral results; patients were enrolled up to 1 year after the fracture occurred (instead of up to 6 weeks after fracture). Because patients were informed that they might be randomized into the placebo group, those with the most severe pain were less likely to enroll in the study. The upcoming results of another randomized, controlled multicenter study (VERTOS [Percutaneous Vertebroplasty Versus Conservative Therapy] II) will soon shed more light on the present contradiction in the literature.
PEARLS AND PITFALLS__________________________________________________
- Strictly lateral and PA views are necessary to avoid inadvertently positioning the needle within or throughout the spinal canal.
- The beveled edge of the intra-pedicular bone needle is turned toward the inner and/or lower margin of the pedicle to avoid passing beyond the cortical bone and damaging intraspinal structures or a nerve root sleeve. It is also useful to direct the tip of the needle within trabecular bone.
- The direction of the pedicle of L5 is often 45 degrees relative to the posterior wall of the vertebral body. With a straight PA trans-pedicular trajectory of the bone needle, the final position of the tip of the bone needle will be lateral to the vertebral body, and cement will be injected into the paravertebral soft tissues.
- The transverse shape of dorsal vertebral bodies may be triangular, and the final position of the tip of the bone needle may easily be lateral to the vertebral body if it is not directed toward the midline. Therefore, study the axial CT or MR images before the procedure.
- The viscosity of the cement is a crucial factor to avoid cement leakage or embolization. If the cement is more liquid than bone marrow, the cement (instead of the bone marrow) will be pushed into the vascular channels and prevertebral veins, and cement emboli may form.
- High-quality fluoroscopy is of paramount importance for a safe cement injection because it makes possible the early detection of cement leakage. If biplanar fluoroscopy is not available, use the lateral view during cement injection and intermittently check the PA view.
Anselmetti GC, Corrao G, Monica PD, et al. Pain relief following percutaneous vertebroplasty: results of a series of 283 consecutive patients treated in a single institution. Cardiovasc Intervent Radiol 2007;30(3):441–447
Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009;361(6):557–568
Evans AJ, Jensen ME, Kip KE, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty retrospective report of 245 cases. Radiology 2003;226(2):366–372
Galibert P, Deramond H, Rosat P, Le Gars D. [Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty]. Neurochirurgie 1987;33(2):166–168
Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8(9):1137–1148
Hasserius R, Karlsson MK, Jónsson B, Redlund-Johnell I, Johnell O. Long-term morbidity and mortality after a clinically diagnosed vertebral fracture in the elderly—a 12- and 22-year follow-up of 257 patients. Calcif Tissue Int 2005;76(4):235–242
Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009;361(6):569–579
Legroux-Gérot I, Lormeau C, Boutry N, Cotten A, Duquesnoy B, Cortet B. Long-term follow-up of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Clin Rheumatol 2004;23(4):310–317
McKiernan F, Faciszewski T, Jensen R. Quality of life following vertebroplasty. J Bone Joint Surg Am 2004;86-A(12):2600–2606
North American Spine Society. Newly Released Vertebroplasty RCTs: A Tale of Two Trials. http://www.spine.org/Documents/NASSComment_on_Vertebroplasty.pdf.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree