2 Cervical Vascular Disease
2.1 Carotid Artery Atherosclerosis: CTA and Ultrasound
2.1.1 Clinical Case
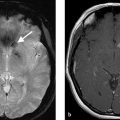
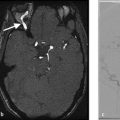
A 56-year-old male with recurrent episodes of left-sided upper extremity weakness which resolve spontaneously over 10 minutes (Fig. 2.1).
2.1.2 Description of Imaging Findings and Diagnosis
Diagnosis
Hypoechoic carotid plaque which is hypodense on CT. On ultrasound, there is a mobile thrombus on the surface of the plaque. Degree of stenosis by North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria is 45%.
2.1.3 Background
Carotid artery stenosis is a well-established risk factor of ischemic stroke contributing to up to 10–20% of strokes or transient ischemic attacks. Many clinical trials over the past 20 years have used measurements of carotid artery stenosis as a means to risk stratify patients. However, with improvements in vascular imaging techniques, such as CTA, MRA, ultrasound and PET/CT, we are now able to risk stratify patients not only on the degree of carotid artery stenosis, but also on biological phenomena, such as plaque composition that can determine how vulnerable the plaque is to rupture and thus ischemic stroke. These imaging techniques are resulting in an emerging paradigm shift that allows for risk stratifications based on the presence of imaging features such as intraplaque hemorrhage (IPH), plaque ulceration, plaque neovascularity, fibrous cap thickness, and the presence of a lipid-rich necrotic core (LRNC).
It is important for the neurovascular specialist to be aware of these newer imaging techniques as they will allow for improved patient risk stratification and outcomes. For example, a patient with a low-grade stenosis, but an ulcerated plaque, may benefit more from a revascularization procedure or aggressive medical management than a patient with a stable 70% asymptomatic stenosis with a thick fibrous cap. In this chapter, we will discuss CTA and US techniques for the assessment of carotid atherosclerotic disease (Table 2.1).
2.1.4 Imaging Findings
Prior studies characterizing components of unstable plaques have demonstrated that CT Hounsfield density can be used to distinguish between LRNC, connective tissue, IPH, and calcifications. Calcifications are easily identified by their high density (mean HU~250). However, there is considerable overlap between the CT densities of LRNC (mean HU~30-50), connective tissue (mean HU~45), and IPH (mean HU~20-30). Macroscopic changes, such as large hemorrhage and large low-density lipid cores, can be easily recognized on MDCT with high interobserver agreement. As a general rule, the lower the density of the plaque, the more likely it is to be vulnerable. MDCT is also very effective at detecting plaque ulcerations, which are defined as crevices within the plaque measuring 2 × 2 mm at minimum.
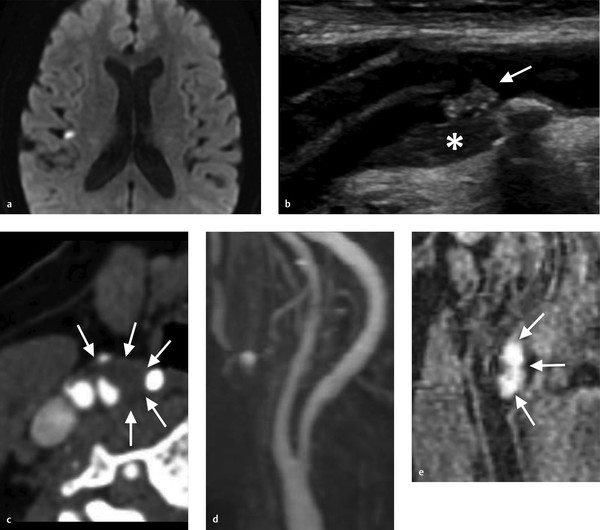
A strong correlation has been demonstrated between sonographic and histopathologic features of carotid plaques using B-mode ultrasound. B-mode carotid ultrasound has high specificity but only moderate sensitivity in identifying ulceration of the plaque surface. Large plaque ulcerations are easily identified as obvious craters within the plaque with reversed or stagnated flow. Determining the presence of ulceration in moderate stenosis patients is important due to the potential for changing the therapeutic approach. Conventional criteria require a size of the concavity greater than 2 × 2 mm and a color Doppler flow signal within the concavity to call something an ulceration (Fig. 2.2).

Plaque echolucency is another strong marker of plaque vulnerability. This is the sonographic equivalent of a LRNC. Plaque echolucency is seen in up to 50% of recently symptomatic plaques compared to less than 5% of asymptomatic plaques. Furthermore, the risk of stroke among patients with echolucent plaques, regardless of the degree of stenosis. In addition, the size of the juxtaluminal hypoechoic area of the carotid plaque is strongly associated with stroke risk.
One critical imaging finding during conventional imaging of carotid disease is examining for the presence of mobile thrombus on the plaque surface. Mobile thrombus is an immediate risk factor for acute ischemic stroke. On ultrasound, it can be identified easily as a piece of plaque or clot that is extending into the lumen and waving in the breeze so to speak. On CTA, it is often seen as a thin hypodensity extending into the internal carotid artery (Fig. 2.3).

Contrast enhanced ultrasound (CEUS) is used primarily to identify neovascularization within carotid plaques. Histologic examinations have demonstrated that plaque enhancement is strongly associated with plaque neovascularity and inflammation. Plaque enhancement is markedly increased in patients with symptomatic plaque and is associated with a higher rate of cardiovascular events in general. Retention of microbubbles on the plaque surface on delayed images has been shown to correlate with plaque disruption and inflammation as macrophages are known to phagocytose this contrast agent. Contrast bubble agents are also used in characterizing the plaque surface. Disruptions in the plaque-lumen border are easily characterized using CEUS. When compared with B-mode and color Doppler ultrasound, along with improved interobserver agreement, CEUS has higher sensitivity, specificity, and accuracy in identifying plaque ulceration.
2.1.5 What the Clinician Needs to Know
Plaque characteristics including hypoechoic plaque on US or hypodense plaque on CT
Presence or absence of plaque ulceration
Presence of free-floating thrombus
Degree of stenosis
2.1.6 High-Yield Facts
Both hypodense plaque (CTA) and hypoechoic plaque (US) are associated with an increased risk of acute ischemic stroke.
CEUS is an emerging tool in the assessment of carotid plaque vulnerability.
Plaque ulcerations are independent risk factors for future ischemic events and can be easily identified on both US and CTA.
Further Reading
[1] Brinjikji W, Huston J, III, Rabinstein AA, Kim GM, Lerman A, Lanzino G. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg. 2016; 124(1):27–42 [2] Gupta A, Gialdini G, Lerario MP, et al. Magnetic resonance angiography detection of abnormal carotid artery plaque in patients with cryptogenic stroke. J Am Heart Assoc. 2015; 4(6):e002012 [3] Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013; 44(11):3071–30772.2 Carotid Vessel Wall Imaging for Atherosclerosis
2.2.1 Clinical Case
A 76-year-old male with recurrent episodes of left-sided upper extremity weakness, which resolved spontaneously.
2.2.2 Description of Imaging Findings and Diagnosis
Diagnosis
Carotid atherosclerotic disease with T1 hyperintense plaque suggesting IPH (Fig. 2.4).

2.2.3 Background
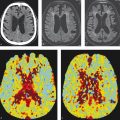
Plaque characterization with MRI has been shown to be a valuable tool in predicting subsequent ischemic events. In a systematic review of nine studies and 779 subjects, one group found that IPH, LRNC, and thinning/rupture of the fibrous cap were associated with hazards ratios of 4.59, 3.00, and 5.93, respectively, for subsequent TIA or stroke. In fact, MRI plaque characteristics have been found to have a stronger association with patient symptomatic status than the degree of stenosis. One large cross-sectional study of 97 patients with 50–99% stenosis found that the presence of a LRNC and thin or ruptured fibrous cap were associated with symptomatic events, whereas the degree of stenosis was not. Size of LRNC and the presence of ulceration have been found to be independent predictors of symptomatic events.
Plaque imaging is also useful in the evaluation of patients with cryptogenic stroke. A number of studies have demonstrated that up to 20–30% of patients with cryptogenic stroke (i.e., stroke of unknown etiology) have a hemorrhagic plaque ipsilateral to the site of the ischemic stroke/TIA. Identifying such lesions early on is important as it can obviate an extensive work-up for other causes of ischemic stroke including arrhythmia and hypercoaguability (Fig. 2.5).

MRI plaque imaging has also been used to identify surgical candidates. Yoshida et al. found that patients with low-grade carotid stenosis with IPH on MRI had high recurrence rates when placed on antithrombotics and statins. Because these patients with IPH were refractory to medical therapy, they elected to proceed with endarterectomy and found that surgery was associated with marked reductions in subsequent ischemic events. MRI plaque imaging has also been found to be useful in identifying revascularization candidates that would be better candidates for carotid endarterectomy (CEA) than carotid artery stenting (CAS) as high intraplaque signal on time of flight (TOF) imaging has been shown to be associated with vulnerable plaque and increased rates of adverse events in patients undergoing CAS, but not CEA.
2.2.4 Imaging Findings
MRI with carotid surface coils is the gold standard in carotid plaque imaging. Multiple contrast weightings are typically performed including fat-saturated T1 pre- and post-contrast, fat-saturated T2, Magnetization Prepared Rapid Gradient Echo, 3D TOF, and gadolinium bolus MRA. Suppression of blood flow signal using a fast spin echo (FSE) sequence with double inversion recovery preparation pulses can result in high contrast between the dark lumen and vessel wall. Pitfalls of surface coil imaging in carotid atherosclerotic disease include improper coil positioning, long scan times, and motion artifact. More recently, some groups have proposed performing carotid plaque imaging with standard neurovascular (or, Head & Neck) coils acquiring plaque imaging with a larger field of view and shorter scan time at the expense of decreased spatial resolution. The images obtained in Fig. 2.4 were performed using a neurovascular coil and a 5-minute MPRAGE sequence.
IPH is the easiest plaque feature to characterize on MRI VWI. Plaque hemorrhage is invariably bright on MPRAGE and T1 weighted sequences with fat suppression. LRNC is characterized by peripheral plaque enhancement with low internal/non-enhancing signal on T1 fat-saturated post-gadolinium imaging (Fig. 2.6). Fibrous cap assessment is often difficult to assess with larger field of view exams; however, surface coil MRI provides an exquisite assessment of fibrous cap status. In cases where there is thick and continuous enhancement of the lumen-plaque interface, the fibrous cap is considered to be intact/thick, whereas in cases where there is discontinuous or disruption of enhancement, the cap is considered to be thin or ruptured (Fig. 2.7). Plaque ulceration is typically defined as a crevice in the plaque of 2 mm or larger and is often indicative of ruptured plaque. A summary of imaging findings of various carotid plaque features on MRI is provided in Table 2.2.


One of the most commonly asked questions about carotid plaque imaging is related to distinguish plaque hemorrhage from dissection. Both will present with high intramural T1 signal. One common means of distinguishing these lesions is location. Carotid plaques are typically located at the carotid bulb, a location that is not typical for dissection. Furthermore, demographically, carotid atherosclerotic disease typically affects older adults, whereas dissections are typically seen in younger patients. Last, carotid atherosclerotic disease is typically associated with disease in the same and other vessels including calcifications, findings which are not typical in acute dissection.
2.2.5 What the Clinician Needs to Know
Plaque characteristics including IPH, lipid-rich core, and thin-ruptured fibrous cap
Whether IPH is juxtaluminal
Presence of free-floating thrombus
Degree of stenosis
2.2.6 High-Yield Facts
IPH is associated with a 5–7× increased risk of acute ischemic stroke.
LRNC and thin-ruptured fibrous cap are also associated with a 3–5× risk of future ischemic stroke.
Plaque characteristics are independent risk factors for acute ischemic stroke-even when controlling for degree of stenosis.
Including a T1 hemorrhage sensitive protocol such as an MPRAGE or 3D-TOF allows for the detection of most hemorrhagic plaques.
Further Reading
[1] Brinjikji W, Huston J, III, Rabinstein AA, Kim GM, Lerman A, Lanzino G. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg. 2016; 124(1):27–42 [2] Gupta A, Gialdini G, Lerario MP, et al. Magnetic resonance angiography detection of abnormal carotid artery plaque in patients with cryptogenic stroke. J Am Heart Assoc. 2015; 4(6):e002012 [3] Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013; 44(11): 3071–30772.3 Spontaneous Carotid Artery Dissection
2.3.1 Clinical Case
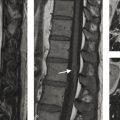
A 58-year-old male in a motor vehicle accident 10 days ago. Now presents with slight right-sided weakness (Fig. 2.8).

2.3.2 Description of Imaging Findings and Diagnosis
Diagnosis
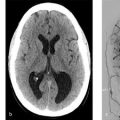
Watershed infarcts in the left hemisphere. Narrowing of the distal cervical ICA secondary to acute carotid dissection.
2.3.3 Background
Cervical carotid artery dissections are a leading cause of ischemic stroke in patients aged 45 years or younger accounting for 20% of all cases in this age group. The incidence of cervical carotid dissection is estimated to be 3/100,000/year. Dissections can be traumatic, or spontaneous. Traumatic dissections, which will be discussed in more details in the blunt cerebrovascular injury (BCVI) chapter, occur from direct injury to the arterial wall from strangulation, direct shock, or motor vehicle accidents. Spontaneous carotid artery dissections occur in the absence of any trauma or minor trauma such as neck manipulation or hyperextension and are often associated with underlying collagenopathies, such as fibromuscular dysplasia (FMD) or Marfan’s syndrome. Typical treatments for non-flow limiting dissections include antiplatelet agents or anticoagulation.
Up to 50% of patients with cervical carotid dissections have histological abnormalities of the collagen tissue in the vessel wall. It is thought that aberrations in collagen III structure are responsible for the arterial wall weakness in these cases. In some cases, it is thought that carotid dissections could be related to an inflammatory etiology, particularly when multiple dissections occur simultaneously. This occurs in the setting of segmental arterial mediolysis.
Pathophysiologically dissections are characterized by a proximal endothelial tear and a further distal intramural hematoma. These hematomas can cleave the arterial wall for a distance. The intramural hematoma can compress the arterial lumen that can result in stenosis or occlusion and subsequent embolic or hemodynamic ischemic stroke. Dilatation can occur when the dissection extends to the subadventitial space and result in pain and cranial nerve compression syndromes including Horner’s syndrome and hypoglossal nerve palsy. Most cervical carotid dissections affect the distal cervical ICA – i.e., where the mobile cervical segment of the ICA enters the foramen lacerum (and thus is immobilized). Usually, the hematoma spares the bulb. Cervical carotid dissections can result in cerebral ischemia through two principal mechanisms: (1) hypoperfusion due to a critical stenosis resulting in watershed distribution infarcts or holohemispheric infarcts in the case of an isolated hemisphere and (2) embolism related to local thrombus formation at the dissection flap/extrusion of intramural clot into the lumen (Fig. 2.9 , Fig. 2.10).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree