3 Cerebral Venous Thrombosis
Summary
While relatively uncommon, cerebral venous thrombosis is a potentially catastrophic condition. Management requires a multidisciplinary expert team and, in rare instances, intervention; the experienced interventional radiologist with expertise in intracranial catheter manipulation may have a role in facilitating rescue. This chapter provides a review of the management of these patients, and provides a case example in which off-label use of devices by an experienced operator led to recanalization with correction of neurologic deficits.
3.1 Introduction
Cerebral venous thrombosis (CVT), which includes the thrombosis of cerebral veins and major dural sinuses, is quite a rare disorder when looked at from a societal perspective. In fact, the annual incidence rate is estimated to be around three to four cases per million. 1 , 2 However, its several risk factors can make certain groups very susceptible, with the implications being rather severe. This chapter will provide a detailed overview of CVT, through analyzing its epidemiology and scope, its manifestation in patients, and the consequent appropriate evaluation and interpretation, as well as treatment options including some procedural tips.
3.2 Clinical Vignette
3.2.1 Patient Presentation
A 79-year-old woman presented with acute confusion and deteriorating levels of responsiveness.
Physical Exam
Physical examination was difficult, as the patient was not following commands.
Imaging
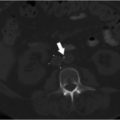
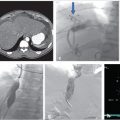
Unenhanced CT of the head demonstrated bilateral parasagittal edema (Fig. 3.1). CT venography showed thrombosis of the midsuperior sagittal sinus.

Treatment Approach
The patient received intravenous heparinization and supportive care but continued to deteriorate over 24 hours. The decision was made to offer catheter-directed intervention.
Specifics of Consent
After a discussion of the risks and benefits, consent for recanalization of the superior sagittal sinus was obtained from the family. A 10 to 20% risk of periprocedural death was discussed, and the off-label use of technology was explicitly disclosed.
Details of Procedure
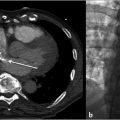
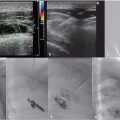
The procedure was performed under general anesthesia. First, right transfemoral arterial access was obtained for transarterial cerebral angiography, which demonstrated occlusion of the midsuperior sagittal sinus, with venous congestion and antegrade venous drainage proximal to the occlusion (Fig. 3.2).

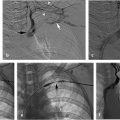
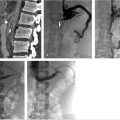
With ultrasound guidance, a retrograde left internal jugular (IJ) access was achieved, as this facilitates navigation of the jugular sigmoid tortuosity. The left IJ was chosen, as direction of flow was from the superior sagittal sinus to the left transverse sinus. A standard 6F vascular sheath was placed, with its tip positioned just into the jugular bulb. This did away with the need for guiding a catheter. Road map guidance was provided by angiography performed via an arterial catheter in the left carotid artery, capturing the road map at the venous phase of the injection. A catheter was advanced through the sheath and navigated to the level of occlusion. A 0.014-inch coronary Hi Torque Cross It wire (Abbott Vascular, Inc., Abbott Park, IL) was then delivered to the level of occlusion through a coronary 4 mm × 20 mm angioplasty balloon. The recanalization wire was used to puncture and cross the dense obstruction and the balloon was passed across the occlusion for venoplasty (Fig. 3.3). Immediate recanalization was achieved and restoration of anatomic retrograde venous drainage established (Fig. 3.4).


Follow-up
Serial CTs over the next few days showed that the venous congestion was improved. The patient’s mentation and mobility improved and mobility was restored over the following few weeks.
3.3 Epidemiology and Scope of Problem
While the annual occurrence of CVT is low, there are clear risk factors that increase the likelihood of specific groups of people developing this condition. The first major risk factor is a patient with a known prothrombotic state. In the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT), 22% of all CVT patients had an inherited prothrombotic state, while 34% were found after presentation to have a constellation of risk factors that resulted in a prothrombotic state. 1 , 3 A prothrombotic state, otherwise known as thrombophilia, may be understood as a tendency to develop clots inappropriately. This disorder can originate based off of genetic factors, acquired factors, or an interaction between the two. Moreover, women who are pregnant, in a post-partum state, or receiving oral contraceptives or other hormonal therapy are also at a higher risk of developing CVT. 4 CVT is also associated with cancer, nephrotic syndrome, localized infections such as otitis, mastoiditis, sinusitis, and meningitis along with systemic infectious disorders, as well as chronic inflammatory diseases like vasculitides and inflammatory bowel disease. Malignancy and hematologic conditions, like sickle cell disease, thrombocythemia, essential thrombocytosis, thrombotic thrombocytopenia purpura, polycythemia, leukemia, and anemia (including paroxysmal nocturnal hemoglobinuria) can also increase a patient’s risk of developing CVT. 1 , 2 , 4 Head trauma, local injury to cerebral sinuses or veins, jugular venous cannulation, neurosurgical procedures, and uncommonly lumbar puncture are possible causes of CVT. 1 , 2 Finally, CVT has a higher frequency in patients under the age of 40. 1
CVT manifests itself pathophysiologically through two major mechanisms. The first is through the increase of the cerebral venous pressure, which leads to decreased perfusion and, ultimately, ischemia, analogous to peripheral compartment syndromes. Moreover, parenchymal hemorrhage can occur secondary to elevated venous pressure. 1 , 2 A second mechanism leading to hemorrhage is decreased absorption of cerebrospinal fluid as a result of the obstructed cerebral sinuses, causing elevated intracranial pressure, which perpetuates the cycle of increased capillary pressure. 4 Distribution of hemorrhage can be posterior temporal if thrombosis is in the transverse sinus, or frontoparietal if the clot is in the superior sagittal sinus.
Mortality from CVT is significant. A meta-analysis of 1,190 patents with this condition showed that there was a 5.6% mean 30-day mortality rate. 5 Transtentorial herniation, often as a result of large venous hemorrhage, was shown to be the primary cause of death during the acute phase of CVT. 5 , 6 Permanent neurological deficits result from thrombosis in 10% of patients at 12 months of follow-up. However, the majority of patients may be expected to return to neurologic function at or near baseline. It is, in fact, remarkable that venous hemorrhage is so much better tolerated than arterial parenchymal bleed. It is notable that patients with this condition have an increased risk of further venous thromboembolism, including deep vein thrombosis and pulmonary embolism, which usually occurs within the first year following the initial thrombotic event. 1
3.4 Patient Presentation and Evaluation
CVT may have various presentations, with related syndromes including intracranial hypertension alone, focal neurologic deficits, seizures, and encephalopathy; whether these syndromes present in isolation or in conjunction is dependent on the extent and location of CVT. 1 The syndrome may present with an indolent course, described as chronic, or may present acutely, with severe or multiple symptThe most common presentation of CVT-caused intracranial hypertension is headache. 1 Indeed, a headache, which can manifest as either localized or generalized and may be aggravated by Valsava maneuvers or position change, 1 , 7 may be present in up to 90% of CVT patients. 1 , 3 . Headaches may also bear resemblance to low CSF pressure headaches. 4 Initial diagnosis of headache induced by CVT is often a migraine. 1 Papilledema and visual complaints can also be noted based off of the aforementioned intracranial hypertension. 1 ] In addition, CVT has also been highlighted as being associated with nausea and vomiting, and may in fact present with intracerebral hemorrhage. 4 Focal neurological deficits are noted in 44% of patients with the condition, with motor weakness in up to 40%. 3 Focal or generalized seizures are observed in 30 to 40% of patients. 8 , 9 Finally, encephalopathy can result from thrombosis of the straight sinus and its branches or from severe cases that can lead to parenchymal hemorrhages, causing herniation, coma, and death. 1 , 2
With respect to the evaluation of patients, there are some fundamental tests or warning flags that should be considered when examining a patient with symptoms that point toward CVT. The AHA/ASA 2011 Scientific Statement on diagnosis and management of CVT recommends imaging of the cerebral venous system in patients with suspected CVT. 10 For suspected CVT patients, head CT is the most frequently performed imaging study. Signs of the condition on CT include hyperdensity in the area of a sinus or cortical vein, and/or filling defects especially in the superior sagittal sinus. 10 Although minor radiation exposure and contrast nephropathy are concerns, computed tomographic venography can provide another reliable method for examining patients with suspected CVT. A major benefit of this approach is that thrombi of heterogeneous densities can be detected, meaning it allows for the diagnosis of subacute or chronic CVT. 1 , 11 The atypical nonarterial distribution of bleeds and the fact that the edema associated with the bleed is disproportionate to the size of the bleed should lead one to consider CVT. MRI of the head combined with MR venography is the most sensitive study for the detection of CVT. 1 , 12 Table 3.1 shows the general signs of acute, subacute, and chronic CVT. MR with T2-weighted imaging and MR venography is recommended by the AHA//ASA 2011 Scientific Statement as the imaging test of choice for the assessment of individuals with suspected CVT. 10 In uncommon cases when there is a high clinical suspicion of the condition, but previous evidence through either MR or CT venography is inconclusive, invasive diagnostic techniques (e.g., cerebral intra-arterial angiography with venous phase imaging and direct cerebral venography) can be used. 10
A complete blood count is recommended to look for polycythemia as an etiologic factor. 4 A decreased platelet count would support thrombotic thrombocytopenic purpura, which is a major risk factor for CVT. Screening for a patient with a prothrombotic state is also highly recommended because of the high frequency of thrombophilias among patients who develop CVT. This screening includes evaluation for the factor V Leiden mutation, prothrombin gene mutation 20210, lupus anticoagulant, anticardiolipin antibodies, hyperhomocysteinemia, and deficiencies of protein C, S, and antithrombin. 1
An elevated D-dimer level supports the diagnosis of the disorder. However, the diagnosis of CVT should not be excluded based on a normal D-dimer level if the patient has a clinical presentation that is otherwise indicative of CVT. 13 , 14 In a study of 239 patients with suspected CVT, D-dimer testing was performed in 98 patients; 9% of the time, a false-positive was noted, while 24% of the time a false-negative was noted. 1 , 8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree