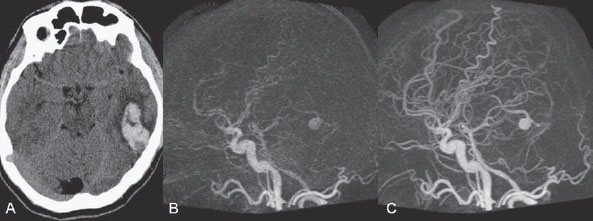
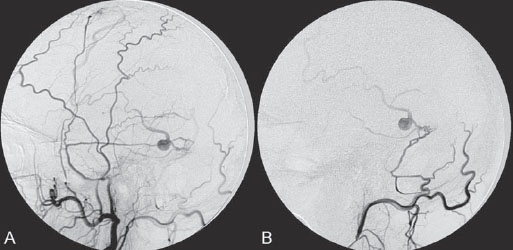
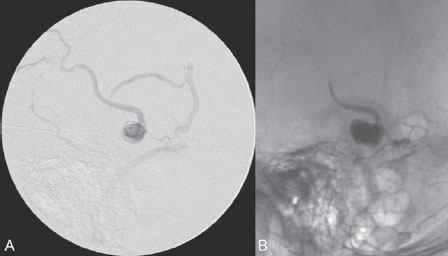
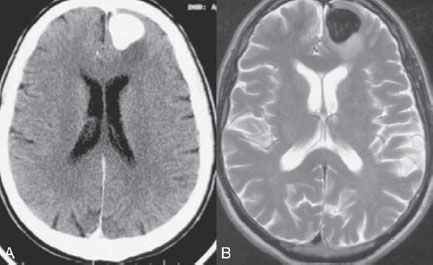
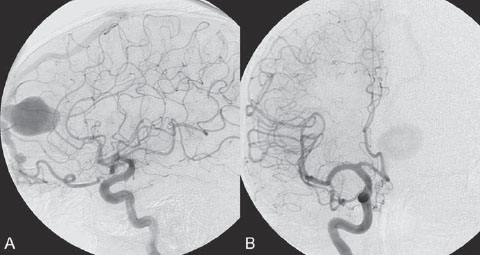
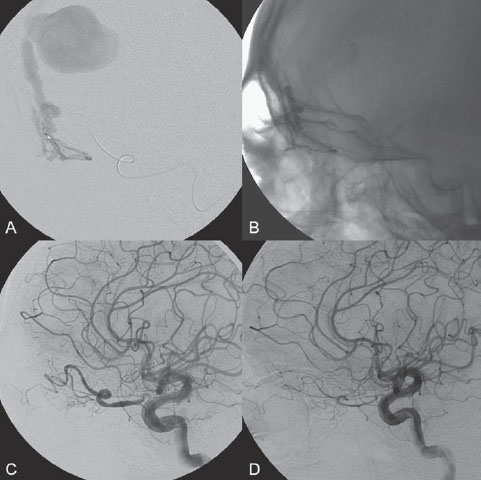
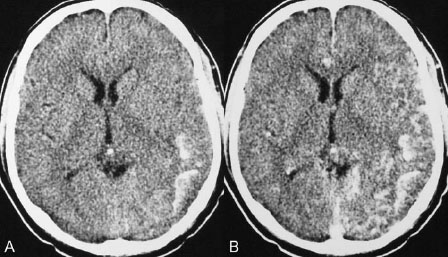
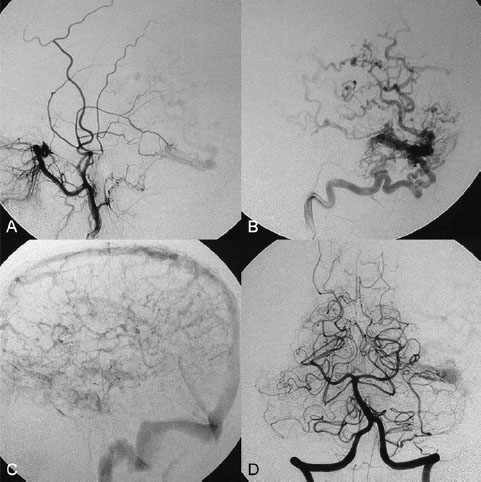
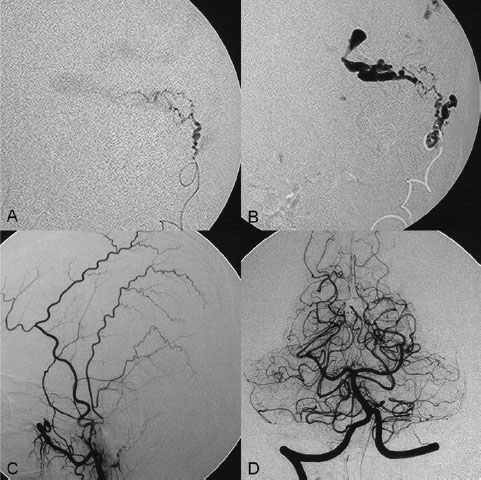
PART III Cranial Dural Arteriovenous Shunts A 44-year-old man presents to the emergency department with the acute onset of severe headaches and dysphasia. His wife reports that the patient has had unusual headaches for the last 3 weeks. Emergency CT is performed, followed by dynamic CTA and angiography. Fig. 23.1 (A) Unenhanced axial CT demonstrates a hyperdense lesion at the left temporal lobe, representing intraparenchymal hemorrhage, with surrounding hypodense brain edema and mild mass effect. Dynamic CTA in (B) early and (C) late arterial phases in lateral view shows early arterial filling of a venous pouch and cortical vein. Plain CT of the brain demonstrated an intraparenchymal hemorrhage in the left temporal lobe measuring 4.5 × 1.8 cm and surrounded by edema. It caused a mild mass effect on the atrium of the left lateral ventricle with no significant midline shift. There was also a small subdural component at the left temporofrontal region. On CTA, an enlarged venous pouch measuring 8 mm in diameter was noted at the posterior aspect of the parenchymal hemorrhage. Dynamic CTA demonstrated early arterial filling of the venous pouch, corresponding to a shunting lesion (Fig. 23.1). Injection of the left external carotid artery (ECA) showed an arteriovenous fistula fed by the petrosquamous branch of the middle meningeal artery and the auricular branch of the occipital artery in the absence of a posterior auricular artery. The two feeding arteries formed a small arterial network just above the level of the sigmoid sinus and then shunted into a pial vein, from which blood refluxed in retrograde fashion into two veins. One of these ran toward the anterior temporal pole and the other ran toward the anterior sylvian fissure, then drained into a frontal cortical vein toward the superior sagittal sinus. This latter vein also filled a branch that ran back to drain into the sigmoid sinus (Fig. 23.2). More importantly, this vein had a venous pouch, which was felt to be responsible for the hemorrhage. The left internal carotid artery (ICA) appeared normal without evidence of venous stagnation or congestion. The remaining territories of both vertebral arteries, the right ICA, and the right ECA were normal. Dural arteriovenous fistula (DAVF) with direct communication into a pial vein having an associated venous pouch (Borden type 3) Following diagnostic angiography, a 5F multipurpose catheter was advanced into the left internal maxillary artery. A flow-directed microcatheter was introduced over a guidewire into the middle meningeal artery and advanced immediately proximal to the shunting zone, as verified by control runs done via the microcatheter. After the microcatheter had been flushed with the glucose solution, a mixture of 1 mL of glue with 1.5 mL of Lipiodol was injected and filled the two veins and venous pouch in antegrade fashion, as well as the arterial network and two feeding arteries in retrograde fashion (Fig. 23.3). The microcatheter was removed, and control runs were performed of both the internal maxillary artery and the occipital artery. These showed no more filling of the fistula. A control run of the left ICA showed unchanged drainage through the left sigmoid artery without evidence of a compromise of the venous flow. Immediately after the procedure, 4 mg of dexamethasone was administered. Fig. 23.2 DSA. (A) Left internal maxillary and (B) left occipital angiograms in lateral view show an AVF supplied by branches of the left middle meningeal artery and left stylomastoid branch originating from the left occipital artery. The AVF drains into an ectatic venous pouch and further drains into two cortical veins. DAVFs are abnormal connections between arteries (that would normally feed the meninges, bone, or muscles but not the brain) and small venules within the dura mater. They account for 10 to 15% of all intracranial AV shunts. The simplest way to classify these lesions is according to whether they do or do not exhibit corticovenous reflux. Although those without corticovenous reflux are benign fistulae (Borden type 1) that almost never lead to neurologic deficits, those with corticovenous reflux may be regarded as malignant fistulae. They are classified as Borden type 2 (if the shunt is directed into the dural sinuses and subsequently into cortical veins) and Borden type 3 (if the shunt is directed immediately into cortical veins). Malignant DAVFs often have an aggressive clinical presentation, with intracranial hemorrhage (e.g., from rupture of venous pouches), seizures, dementia, alteration of consciousness, and focal nonhemorrhagic neurologic symptoms due to venous congestion. Given the high risk for hemorrhage, complete treatment of all malignant DAVFs must be carried out. In most centers, endovascular management is the therapy of choice. Fig. 23.3 (A) Superselective microcatheter injection within the left middle meningeal artery branch and (B) plain radiography demonstrate the glue cast filling the venous pouch and proximal vein after embolization. Published Literature on Treatment Options Given that DAVFs with cortical venous reflux carried an annual risk of more than 8% for hemorrhage and 6% for nonhemorrhagic deficits and an annual mortality rate of 10% in the series reported by van Dijk et al from our group, curative treatment is deemed a necessity and should be obtained in a timely fashion. If endovascular treatment is unsuccessful, then surgical treatment should be performed during the same hospital admission. Both surgical and endovascular treatment options follow the same principle (i.e., to occlude the distal arterial site and the proximal venous outflow), and high success rates with low rates of procedure-related morbidity and mortality have been reported. PEARLS AND PITFALLS__________________________________________________ Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995;82(2):166–179 Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995;194(3):671–680 Davies MA, TerBrugge K, Willinsky R, Coyne T, Saleh J, Wallace MC. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg 1996;85(5):830–837 van Dijk JM, terBrugge KG, Willinsky RA, Wallace MC. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 2002;33(5):1233–1236 A 63-year-old man presents to the emergency department with the acute onset of seizures. Emergency CT and MRI are performed, followed by angiography. Fig. 24.1 (A) Contrast-enhanced CT and (B) T2-weighted MRI demonstrate a venous pouch with surrounding brain edema. A small flow void adjacent to the venous pouch, corresponding to a dilated cortical vein, is noted on the T2-weighted image. Contrast-enhanced CT of the brain demonstrated a well-defined, intensely enhancing vascular structure at the left frontal lobe, consistent with a venous pouch (Fig. 24.1). Surrounding hypodensity indicated brain edema. A T2-weighted image demonstrated similar findings. The tubular flow-void structure adjacent to the venous pouch, corresponding to a dilated cortical vein, and the brain edema were better seen than on the CT scan. Injection of the left internal carotid artery (ICA) showed an arteriovenous fistula (AVF) at the level of the anterior cranial fossa floor, supplied mainly by the ethmoidal branches of the left ophthalmic artery (Fig. 24.2). There was also minimal supply from branches of the right ophthalmic artery. No significant arterial supply from the external carotid artery (ECA) branches was seen, although the venous pouch was faintly opacified through indirect supply from the nasoethmoidal branches of the left facial artery (not shown). There was direct drainage into the left frontal cortical vein, with a large venous pouch, and further drainage into the superior sagittal sinus. Fig. 24.2 DSA. (A) Left ICA injection in lateral view and (B) right ICA injection in AP view show an AVF at the anterior cranial fossa, supplied mainly by the ethmoidal branches of (A) the left ophthalmic artery with minimal supply from (B) the right ophthalmic artery. There is direct drainage into a left frontal cortical vein, which harbors a large venous pouch, before drainage into the superior sagittal sinus. Ethmoidal dural arteriovenous fistula (DAVF) with a direct communication into a cortical vein and an associated venous pouch (Borden type 3) Following diagnostic angiography, a 5F multipurpose catheter was advanced into the left ICA. A flow-directed microcatheter was introduced over a micro-guidewire into the left ophthalmic artery and advanced to a point immediately proximal to the shunting zone. Control runs were done by hand injections via the microcatheter to confirm the position (Fig. 24.3). After the microcatheter had been flushed with the glucose solution, a mixture of 1 mL of glue and 2 mL of Lipiodol was injected, penetrating the shunt zone and filling the proximal draining vein without any reflux of the glue. The microcatheter was removed, and control runs were performed of both ICAs and both ECAs, confirming complete closure of the fistula. Fig. 24.3 (A) Superselective injection and (B) glue cast following embolization show glue filling the proximal venous segment. Control left ICA angiograms (C) immediately after and (D) 3 months after the procedure confirm complete obliteration of the fistula. Note the remodeling of the ophthalmic artery after embolization. DAVFs at the anterior cranial fossa are also known as “ethmoidal” DAVFs. Such DAVFs are rare and can be classified among the DAVFs of the lateral epidural type. Other places where lateral epidural DAVFs are found include the spine (the classic location), foramen magnum, falx (vein of Galen), tentorium (basal vein of Rosenthal and superior petrosal sinus), and cortical veins. Arterial supply to ethmoidal DAVFs is characteristically from the ethmoidal branches of the ophthalmic artery, and venous drainage is through the frontal cortical veins before a sinus (therefore, ethmoidal DAVFs are always Borden type 3 lesions). They are often associated with venous pouches, which frequently rupture and result in intracranial hemorrhage. Transarterial embolization is the treatment of choice in our institution, followed by open surgery when embolization fails to cure the lesion. Published Literature on Treatment Options Surgery is the most common type of treatment in previous case series, usually with a 95 to 100% cure rate. Operations are typically performed via a frontal or bifrontal craniotomy to identify the durabased feeding arteries and enlarged draining cortical veins. Subsequent disconnection of the fistula on the venous side is then performed, with a low risk for stroke and blindness. In the past years, an increasing number of case series have reported the successful use of endovascular treatment. In the report by Agid et al, the success rate for transarterial embolization through the ophthalmic artery was ~60%. Embolization through indirect branches of the ECA is usually not successful. Although transvenous approaches have been reported, they are technically more challenging, requiring navigation of the microcatheter to the anterior superior sagittal sinus and then selectively into the draining cortical vein. Coiling of this vein must be done as close as possible to the fistulous site and proximal to the venous pouch to avoid a catastrophic hemorrhage. PEARLS AND PITFALLS__________________________________________________ Abrahams JM, Bagley LJ, Flamm ES, Hurst RW, Sinson GP. Alternative management considerations for ethmoidal dural arteriovenous fistulas. Surg Neurol 2002;58(6):410–416, discussion 416 Agid R, Terbrugge K, Rodesch G, Andersson T, Söman M. Management strategies for anterior cranial fossa (ethmoidal) dural arteriovenous fistulas with an emphasis on endovascular treatment. J Neurosurg 2009;110(1):79–84 Lawton MT, Chun J, Wilson CB, Halbach VV. Ethmoidal dural arteriovenous fistulae: an assessment of surgical and endovascular management. Neurosurgery 1999;45(4):805–810, discussion 810–811 Lv X, Li Y, Wu Z. Endovascular treatment of anterior cranial fossa dural arteriovenous fistula. Neuroradiology 2008;50(5):433–437 A 49-year-old man presented 2 years previously with complex partial seizures, which were controlled with medication. Now his seizures are worsening despite increases in his seizure medication. There are no neurologic deficits on clinical examination. A CT scan is performed at an outside hospital, and the patient is referred to our institution for angiography and treatment. Fig. 25.1 (A) Unenhanced and (B) post-contrast CT scans demonstrating subcortical calcification at the level of the left temporo-occipital lobe, with enhancing vessels along the cortical surface of the left cerebral hemisphere. Unenhanced CT scan showed subcortical calcification involving the left temporo-occipital lobe. After the administration of contrast, prominent enhancing vessels along the cortical surface of the left cerebral hemisphere were noted, suggestive of a dural arteriovenous fistula (DAVF) with cortical venous reflux (Fig. 25.1). Injection of the left external carotid artery (ECA) showed early filling of an irregular left transverse sinus segment, supplied by branches of the left occipital artery and additional supply from the left middle meningeal artery and left posterior meningeal artery. Thrombosis of the proximal left transverse sinus and distal left sigmoid sinus created an isolated sinus segment, resulting in reflux of the venous drainage into the left occipital cortical veins. A pseudophlebitic appearance of the cortical veins in the venous phase of the left internal carotid artery (ICA) injection was noted, suggesting venous congestion (Fig. 25.2). Fig. 25.2 DSA. (A) Left internal maxillary and (B) left occipital angiograms in lateral projection demonstrate an AV shunt supplied by branches of the left middle meningeal artery and left occipital artery and draining into an irregular venous segment of the left transverse sinus. Occlusion of the left proximal transverse sinus and distal sigmoid sinus segments is noted, causing cortical venous reflux into the left occipital veins. (C) Left ICA angiogram in late venous phase shows a pseudophlebitic pattern of the cortical veins with stagnation of the contrast, corresponding to venous congestion. Additional supply to the DAVF from the left posterior meningeal artery of (D) the left VA is also noted. Aggressive DAVF of the left transverse sinus associated with an isolated sinus (Borden type 2) Following diagnostic angiography, a 5F multipurpose catheter was advanced into the left occipital artery (Fig. 25.3). An over-the-wire microcatheter was introduced over a micro-guidewire into the dural branches originating from the occipital artery in a wedged position as close to the shunt zone as possible. Control runs were done via hand injection through the microcatheter to verify the position. The microcatheter was then flushed with the glucose solution, followed by the injection of glue at 50% concentration in an attempt to fill the venous pouch. The procedure was repeated twice, both times through branches of the occipital artery. Following the second glue embolization, filling of the isolated sinus segment was no longer seen on control angiograms. Follow-up angiogram at 3 months confirmed the complete closure of the DAVF. DAVFs are abnormal AV shunts located within the dura mater comprising the walls of the dural sinuses. The transverse and sigmoid sinuses are the most common location, accounting for ~30 to 50% of all DAVFs. Lesions in this location are typically benign (Borden type 1, without cortical venous reflux). Exceptions are cases with extensive thrombosis of the venous outflow and those with high-flow shunts, which may result in malignant dural AV shunts with cortical venous reflux (Borden type 2). In patients in whom the involved dural sinus is still patent, cure by endovascular means is difficult because in most instances the patent dural sinus is still a normal pathway for brain drainage, and its closure may result in venous infarction. Fig. 25.3 (A) Hand injection through a microcatheter wedged within a branch of the left occipital artery in AP view shows filling of the isolated sinus segment, followed by (B) the glue injection. (C) Follow-up ECA and (D) right VA angiograms at 3 months show complete closure of the fistula.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree