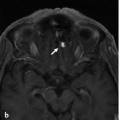
Fig. 32.1 Unenhanced axial CT (a,b) demonstrates an area of white matter hypodensity at the high left frontoparietal region. Contrast-enhanced axial (c) and coronal CT (d) reveal abnormal vessels along the cortical sulci and periventricular white matter in bilateral cerebral hemispheres. These findings are suggestive of venous hypertension with venous infarction, likely related to an underlying dAVF and/or venous thrombosis.
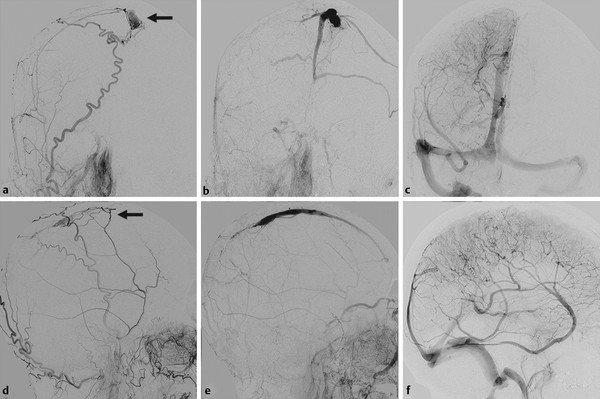
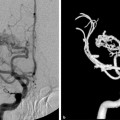
Fig. 32.2 Right external carotid artery (ECA) angiogram in arterial (a,b) and venous (c,d) phases reveals a dAVF at an isolated SSS (arrows), with thrombosis of the posterior aspect. The shunt is mainly supplied by branches of the middle meningeal artery with reflux into the cortical veins. Right internal carotid artery (ICA) angiogram in late venous phase (e,f) shows no filling of the SSS, with rerouting of the drainage through the transmedullary veins into the deep venous system and vein of Labbé to the transverse sinus.
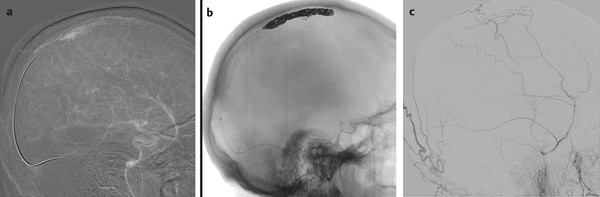
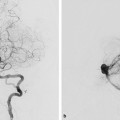
Fig. 32.3 Subtraction roadmap image in lateral view (a) shows the position of the guidewire through the thrombosed segment of the SSS. Skull radiography in lateral view (b) reveals the coil meshwork, and right ECA angiogram in lateral view postembolization (c) demonstrates complete obliteration of the dAVF. The patient experienced significant improvement of his neurological symptoms.
32.1.3 Diagnosis
Malignant-type superior sagittal sinus (SSS) dural arteriovenous fistula (dAVF) with occlusion of the posterior SSS and rerouting of the shunt into transmedullary veins.
32.2 Embryology and Anatomy
In a 4-week embryo (4 mm), three meningeal venous plexuses (anterior, middle, and posterior) can be identified draining into the primitive head vein. By the 14-mm embryo stage, the primordium of the SSS, the sagittal plexus can be appreciated, formed from the midline coalescence of the anterior and middle venous plexuses. The numerous epidural plexuses, by confluence of their lumen, will evolve to become what is in adult life seen as the SSS. The development of the brain and skull base leads to the adult configuration of the SSS by the 50-mm stage (9-week fetus), in which the SSS joins with the straight sinus to drain into the torcular herophili. During this development, asymmetrical growth of the SSS predisposes it to drain freely into one side more than another, usually to the right transverse sinus; the straight sinus typically prefers the left transverse sinus.
Several variations of the SSS may be encountered. The most extreme form includes absence of the posterior SSS and drainage of the anterior portion of the brain through an anterior-dominant sinus pericranii via a midline vein at the forehead. If no posterior outlet for the SSS exists, occlusion of this vein must be avoided to prevent cerebral venous infarctions. Posteriorly, there may be a high division of the SSS, with two separate channels going to each transverse sinus.
This so-called duplication of the SSS is likely related to incomplete midline fusion of the plexuses that form the SSS. In its incomplete form, if the dural sinuses retain some of their embryologic plexiform pattern, septations within the SSS may be seen (more frequently in the posterior aspect; ▶ Fig. 32.4).
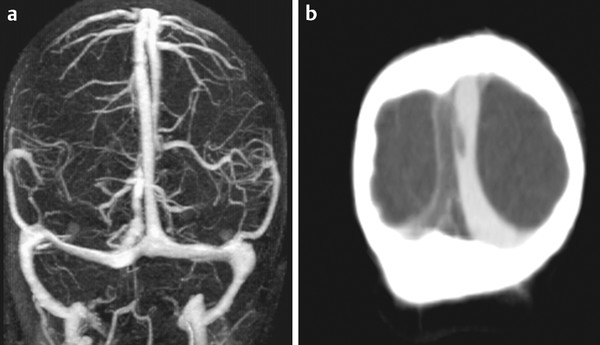
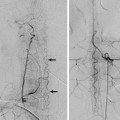
Fig. 32.4 Venous 3D phase contrast MRA (a) and contrast-enhanced CT venography (b) in the same patient demonstrate thrombus within the right channel of the SSS close to the torcular. It is best seen on CT and is not as well appreciated on magnetic resonance because of the compartmentalization of the sinuses.
Cavernous spaces located within the walls of the dural sinuses, including the SSS, have been reported. On occasion, these spaces may also bulge into the lumen of the dural sinus. Small arteries from the middle meningeal branches are also observed to terminate within these cavernous spaces; these may play a role in the formation of dAVFs and are presumably also related to the dural sinus malformations seen in childhood.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree