4 Acute Deep Venous Thrombosis: Sporadic and Compressive
Summary
Acute deep venous thrombosis (DVT) may be sporadic, or can result from compressive phenomena, which are recognized as distinct anatomic syndromes. The management of DVT in the acute stage is increasingly of interest to interventional radiologists, as improvement in customized devices has allowed for better outcomes of intervention. This chapter reviews the evaluation of patients with acute DVT, and discusses the management of patients appropriate for pharmacomechanical catheter-directed therapy, with a discussion of management of underlying compressive syndromes. The reader can be expected to become familiar with relevant anatomic variants and their management, as well as developing an understanding of the pharmacologic and device-based interventions available for this patient population. A discussion of the details of procedural management, as well as postprocedural follow-up, is included in the chapter.
4.1 Introduction
Deep venous thrombosis (DVT) is a common medical problem with varying underlying etiologies and multiple immediate and long-term sequelae. Nearly 600,000 hospitalizations in the United States are attributable to DVT annually. Advances in the detection and treatment of DVT hold the potential to significantly impact a patient’s quality of life both in the acute setting and over time. Herein, the evaluation and treatment of DVT is reviewed, including a review of two common syndromes that predispose to DVT: iliac vein compression syndrome (commonly known as May–Thurner syndrome [MTS]) and Paget–Schroetter syndrome (PSS), also known as axillosubclavian venous effort thrombosis.
4.2 Sporadic Deep Venous Thrombosis
DVT most commonly affects veins of the lower extremities; however, the veins of the upper extremities, neck, central thorax, pelvis, and inferior vena cava (IVC) may also be involved. While the visceral and portal venous systems are located deep within the body, the term DVT is generally not used to describe thrombosis in these vessels. In the upper and lower extremities, the deep veins accompany their similarly named arterial counterparts. Symptoms manifest as a direct result of venous obstruction, or due to venous insufficiency as a result of valvular incompetence and decreased venous compliance, both of which are common in recanalized veins previously affected by DVT.
4.2.1 Case Vignette
Patient Presentation
A 46-year-old man presents with new right lower extremity swelling, chest pain, and shortness of breath.
Physical Exam
The patient presented with 2–3 right lower extremity edema, cyanosis, and warmth to the knee. Prominent bulging superficial veins were present in the upper thigh. He exhibited normal lower extremity motor and sensory exams, but displayed pain with deep palpation and during forced dorsiflexion of the right ankle (positive Homan’s sign). He was hemodynamically stable. Chest pain was noted on deep inspiration.
Imaging
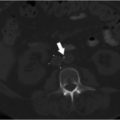
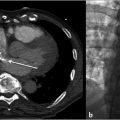
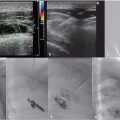
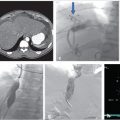
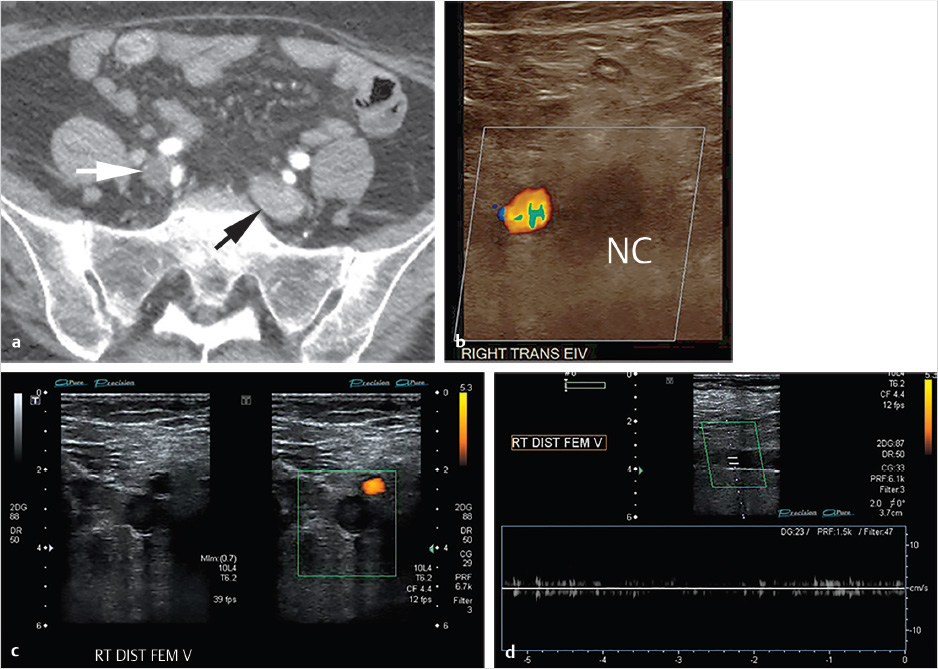
CT of the chest and abdomen with contrast revealed acute right lower lobe pulmonary emboli and right lower extremity DVT extending to the right common iliac vein (Fig. 4.1a). Lower extremity duplex ultrasound revealed hypoechoic occlusive material within noncompressible lower extremity veins extending from the external iliac to the central aspect of the femoral vein consistent with acute DVT (Fig. 4.1b; Fig. 4.1c; Fig. 4.1d). The right popliteal vein and the left lower extremity veins were patent.
Specifics of Consent
Given his symptoms, the patient requested aggressive treatment for his DVT. The risks of venous injury, bleeding, infection, pulmonary embolism (PE), and recurrent thrombosis were all discussed and the patient agreed to proceed.
Details of Procedure
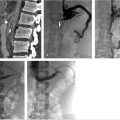
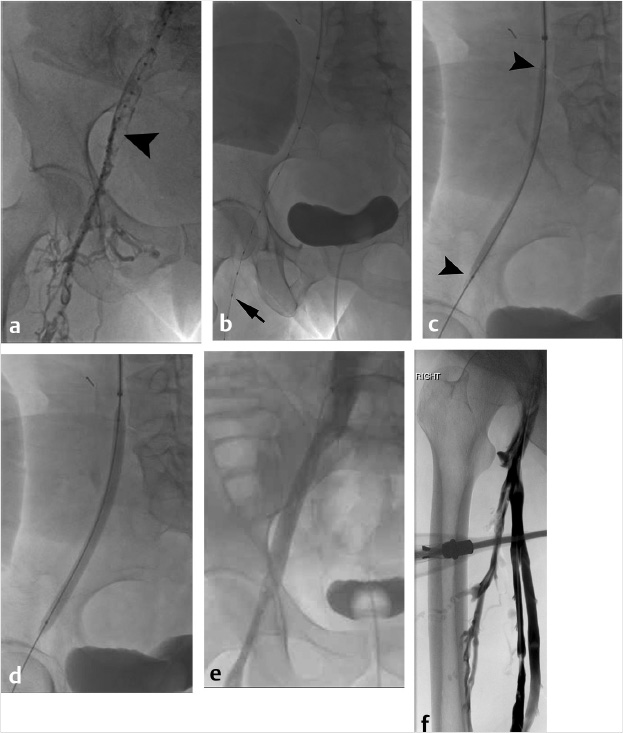
At the time of initial evaluation, anticoagulation was initiated with intravenous heparin. Placement of an IVC filter was discussed for further pulmonary embolus prophylaxis. Right internal jugular vein was accessed under ultrasound guidance and IVC venography was performed prior to deployment of an infrarenal IVC filter. The filter sheath was exchanged for a 9-French sheath, which was positioned below the filter in the IVC. A hydrophilic wire was navigated peripheral to the common femoral vein and right lower extremity, and pelvic venography was performed. Occlusive thrombus was noted extending from the right common femoral to the common iliac vein (Fig. 4.2a). A multiside hole thrombolysis catheter was laid along the extent of thrombus (Fig. 4.2b). Overnight catheter-directed thrombolysis (CDT) was initiated with alteplase at 1 mg/h. By the next day, the thrombus load had reduced significantly. Venoplasty was undertaken with a 10-mm balloon across the length of the vein (Fig. 4.2c; Fig. 4.2d). The thrombus completely resolved the following day. Final venography demonstrated brisk anterograde venous flow without residual thrombus (Fig. 4.2e; Fig. 4.2f).
Follow-up
At the conclusion of the procedure, compression stockings were applied and the patient was continued on therapeutic anticoagulation. At discharge 2 days later, his lower extremity swelling had considerably improved. At a follow-up clinic visit 4 weeks after discharge, the patient had returned to baseline. A same-day duplex ultrasound revealed no evidence of recurrent DVT.
4.3 Epidemiology and Scope of the Problem
As many cases of DVT are likely subclinical, precise epidemiologic data are difficult to obtain. The incidence is estimated at 45 to 117 cases per 100,000 person-years for isolated DVT without PE. DVT is often combined with PE into a larger category known as “venous thromboembolism” (VTE) for statistical tracking; the estimated incidence of VTE is 104 to 183 cases per 100,000 person-years. In the United States, VTE affects approximately 0.96 to 3.0 per 1,000 individuals per year, which equates to over 1 million patients in this country annually. 1 The vast majority of cases are diagnosed in older patients, with the incidence rising dramatically over the age of 60. The overall incidence of VTE ranges from 1 per 10,000 at age 20 up to 10 per 1,000 by age 80. DVT is diagnosed in men slightly more often than in women (1.2:1 ratio), although under the age of 45, women are more commonly affected. Isolated DVT without PE is more common in younger patients, with concomitant PE seen more frequently beginning in the seventh decade. 2
The high incidence and frequent morbidity associated with DVT burdens the health care system significantly. DVT is a frequent complication of patient hospitalization; it is the second most common reason for prolonged hospital stays, and is the third most common cause of excess hospital-related mortality. More than half of all cases of DVT are encountered in the hospital setting, with 25% occurring in the perioperative setting. The average length of stay for patients with isolated DVT ranges from 4.9 to 7 days, increasing to 7.4 to 9 days in patients with concomitant PE. Readmissions are common in this population and subsequent stays are often longer than the initial admission. Current cost estimates for a primary admission for DVT range from $3,000 to $6,000 and are significantly higher for readmissions for the same problem. 1 However, the economic burden does not end at the time of discharge. Recurrent thromboembolic disease is common, and therefore frequent follow-up and long-term anticoagulation are needed to prevent a repeat event. Data from 2004 suggest the total cost of care for a first-event DVT without PE exceeds $33,000, taking all factors into account; this cost has undoubtedly increased. 3
Unfortunately, even with successful management of an initial DVT, long-term sequelae are common. Aside from recurrent DVT, patients may develop lower extremity venous insufficiency, with an estimated 25 to 50% leading to formation of varicose veins, skin changes and ulcerations, and chronic debilitating symptoms associated with postthrombotic syndrome (PTS). The societal cost, including lost productivity, personal disability, and long-term health care, can be staggering.
4.3.1 Patient Presentation and Evaluation
A detailed history and physical examination are critical during the initial evaluation of a patient with suspected DVT. The most common presenting symptoms, regardless of location, are edema and pain. The modified Wells’ criteria for DVT serve as a tool to guide clinicians as to the likelihood of DVT in a patient presenting with symptoms suggestive of the diagnosis. 4 At least one component of the classic “Virchow’s triad”—venous stasis, vessel wall injury, and hypercoagulability—is typically identified through a thorough history and laboratory evaluation. A discussion of these three variables follows.
Venous stasis refers to decreased flow through the deep veins, which occurs with decreased extremity movement. DVT can be caused by a prolonged bedbound state, immobilization, limb paresis, or long-distance travel. Trauma patients are particularly susceptible to several of these factors and are at high risk for DVT. Pregnant patients experience compression on their pelvic veins, preventing venous return. Morbidly obese patients are also at an increased risk, likely due to a combination of factors.
Vessel wall or endothelial injury can refer to a number of perturbations to the normal antithrombotic nature of venous endothelium. When the vein wall is damaged, prostaglandin and nitric oxide levels are decreased, thromboplastin is released, and subendothelial collagen and von Willebrand factor are exposed—each encouraging platelet adhesion to the site of injury. Inflammatory cytokines, including interleukin-1 and tumor necrosis factor alpha, are released at the site of vessel wall damage, causing the synthesis and release of tissue factor. 5 Platelet adherence stimulates the release of thromboxane A2 and ADP, which cause further recruitment and aggregation of circulating platelets, bound to one another by fibrinogen. Aggregated platelets and polymerized fibrin combine to form thrombus. Factors that can cause endothelial dysfunction or damage to the vessel wall and lead to this cascade include direct venous trauma (including venipuncture and central venous access placement), hypertension, turbulent flow (particularly across valves, which is more pronounced in valves damaged by prior DVT or phlebitis), circulating toxins from cigarette smoke, radiation injury, bacterial endotoxins, hypercholesterolemia, and hyperhomocysteinemia.
Hypercoagulability can occur in a variety of inherited and acquired conditions, each carrying varying degrees of increased risk for the development of DVT. Deficiencies in protein C, protein S, and antithrombin, activated protein C resistance, prothrombin G20210A, and factor V Leiden mutations, antiphospholipid antibody syndrome, elevated plasma levels of factor VIII or fibrinogen, use of oral contraceptives, pregnancy, and other hormonal factors are all causes of increased thrombus generation. Hypofibrinolysis is seen in patients with elevated levels of the primary inhibitor of tissue plasminogen activator (PAI-1), as well as in patients with elevated levels of thrombin-activatable fibrinolysis inhibitor (TAFI); the end result is an inability to break down early foci of thrombus, leading to DVT. Several other conditions including cancer (particularly myeloproliferative neoplasms), paroxysmal nocturnal hemoglobinuria, Behçet’s disease, and multiple autoimmune disorders have all been found to have an association with increased DVT. 6 Evaluation for thrombophilia may be warranted, particularly in unprovoked DVT in younger patients. Extensive panels of tests to determine hypercoagulable states exist to help identify the most common conditions. This topic is discussed in detail in Chapter 2 of this book.
On physical examination, focal or diffuse edema and pain with deep palpation are often present. When present, the classic physical exam finding of pain upon dorsiflexion of the calf (Homan’s sign) is helpful in the diagnosis of lower extremity DVT, but is only noted in 50% of cases. Upper or lower extremity DVT may cause erythema or bluish discoloration of the skin at and upstream from the level of the thrombus. The affected limb is typically warm, and prominent superficial veins are common. The presence of a central line on the same side as the patient’s symptoms should raise suspicion for DVT, whether in the upper or lower extremity.
Initial noninvasive testing consists primarily of a duplex ultrasound evaluation. Sonographic findings of acute thrombosis include noncompressibility of the vein, venous expansion, absence of flow on spectral waveform analysis and color Doppler imaging, increased flow through collateral veins, and hypoechoic intraluminal filling defects; in hyperacute thrombosis, the thrombus may even be sonolucent. Diminished augmentation of flow with distal tissue compression is often noted when evaluating segments central to the level of the DVT. Venous Doppler waveforms normally exhibit phasicity, which is a result of both cardiac and respiratory variation; examination of a venous segment peripheral to an area of occlusive thrombus will reveal partial or complete loss of phasicity. 7 In the upper extremities, venous waveforms also characteristically demonstrate significant transmitted cardiac pulsatility, which is lost in the setting of central occlusion.
Computed tomography venography (CTV) and magnetic resonance venography (MRV) both provide excellent evaluation of the venous system when imaging is acquired following a sufficient postinjection delay (~120 seconds). 8 These modalities are generally reserved for thoracic and abdominopelvic evaluations in suspected central DVT, or to identify/exclude central venous compression syndromes. Findings of DVT on both CTV and MRV include vein expansion, nonopacification of involved vessels, luminal filling defects, vessel wall enhancement, perivascular edema, and increased flow through collateral veins. On noncontrast CT, increased attenuation can often be seen within the lumen of thrombosed veins. On MRI, signal characteristics within the lumen of thrombosed vessels vary significantly depending on the acuity of the DVT, with T1 and T2 signal in general increasing over time after the first 1 to 2 days. Noncontrast time-of-flight MRV sequences are also available for patients who cannot receive contrast, with thrombosed vessels showing decreased or absent flow-related signal.
Since the introduction of heparin in the 1930s, systemic anticoagulation has been the primary method of treatment for DVT. 9 Anticoagulants prevent propagation of thrombus and decrease the risk of central embolization; however, in general, these medications lack sufficient thrombolytic effect for active thrombus dissolution. 10 Over the past two decades, minimally invasive techniques including CDT, mechanical thrombectomy, and pharmacomechanical thrombectomy have emerged, allowing more rapid clearing of thrombus. Multiple factors are used to determine which therapeutic strategy may best be indicated for a particular patient; however, a lack of sufficient long-term evidence necessitates a highly individualized algorithm. Factors to consider when deciding which patients will benefit from more aggressive therapy for DVT include potential for development of PTS, the risks of hemorrhagic complications, and the presence of (or risk for) PE. While PTS and PE may lead to decreased quality of life, cardiopulmonary compromise, or even death, the risks of interventional DVT therapy are not insignificant, and therefore an intervention is currently considered to be unwarranted in a patient with an incidentally discovered thrombosis and/or minimal or absent symptoms. 11 Conversely, patients with severe acute symptoms should be strongly considered for aggressive therapy (especially those with phlegmasia cerulea dolens, representing peripheral ischemia due to venous thrombosis, compartment swelling, and arterial compression). The patient’s age should also be taken into account, given the risk of long-term complications of DVT (particularly iliofemoral DVT) increases over time. A short life expectancy is a relative contraindication to catheter-directed therapy; however, this assessment should take into account the patient’s activity level and quality of life prior to the diagnosis of DVT.
The location of thrombosis is also an important data point when weighing the risk–benefit ratio of aggressive versus conservative therapy for DVT management. Approximately 4% of all documented DVTs involve the upper extremities, of which more than 75% are considered “secondary” or “provoked” (e.g., catheter-related). 12 The incidence of PE in these patients is small; however, upper extremity PTS is approximately 15%. 13 Patients with brachiocephalic and subclavian vein involvement tend to be the most symptomatic and have the highest risk for upper extremity PTS.
In cases of lower extremity DVT, it is relatively uncommon to develop only a single level of thrombus; when present, these clots tend to produce mild symptoms or are completely asymptomatic. Isolated caval thromboses are the least likely to produce symptoms in isolation, but are the most likely to produce PE. The risk of both PE and PTS decreases in more distal segments, such that it is relatively rare to develop either from calf thrombosis alone, particularly when involving only a single calf vein. The iliac and popliteal veins appear to comprise the most critical levels, with occlusions involving these segments producing the most symptoms in isolation and contributing the greatest risk of subsequent PTS; when either is combined with multilevel thrombosis involving additional segments, symptoms and risk of developing PTS increase significantly. 14 , 15
Contraindications to long-term anticoagulation in symptomatic patients may not only represent an indication for IVC filter placement to prevent pulmonary embolization in some circumstances, but also include the need for DVT thrombectomy and possibly thrombolysis. A history of frequent falls is a common exclusion therapy for long-term anticoagulation, but does not necessarily preclude thrombolytic therapy during which patients are generally confined to bed and housed in hospital units with higher levels of observation and care. Standardly accepted contraindications to systemic thrombolysis include recent surgery, trauma or childbirth, intracranial lesions, history of hemorrhagic stroke (ever) or ischemic stroke (within the past year), active bleeding, or uncorrectable coagulopathy. In these patients, mechanical thrombus removal may be considered, with pharmacomechanical adjunctive measures employed on a case-by-case basis.
4.3.2 Preparation for Procedure
The initial decision when treating a patient with DVT is whether treatment can be completed in a single session or if overnight CDT will be required. In general, longer segment DVT, with a greater propensity for creating clinically relevant PE, is more commonly treated with CDT, while shorter segment or isolated segment thrombosis may be treated with either CDT or pharmacomechanical thrombectomy. CDT carries with it multiple requirements that may be unexpected to the patient: dietary, activity, and limb restrictions during the infusion, ICU admission, the insertion of a urinary catheter, and procedures on multiple days. These should be discussed in detail during the consent process in order to appropriately set expectations. If thrombolysis is expected, placement of multiple secure peripheral intravenous accesses and insertion of a urinary catheter is recommended prior to initiation of thrombolysis. Preprocedural assessment evaluating the safety of moderate sedation should be performed; involvement of anesthesiology support should be considered in all patients with American Society of Anesthesiology Physical Status (ASA-PS) scores ≥4 or Mallampati scores of ≥3. 16
Baseline laboratory studies are required prior to initiating DVT intervention. A complete blood count (CBC) should be obtained; a baseline hemoglobin ≥ 8 g/dL is advised, and can be compared to future values for evidence of bleeding. For most operators, a platelet count ≥ 50 K/µL is considered a safe threshold for venous puncture and lysis initiation. Determination of coagulation status, specifically partial thromboplastin time (PTT) and prothrombin time (PT)/international normalized ratio (INR), is also necessary prior to intervention, particularly if pharmacologic thrombolysis is anticipated. PTT ≤ 30 seconds and INR ≤ 1.5 are typical thresholds prior to initiating thrombolysis, as significantly elevated values of either are predictive of increased bleeding risk during thrombolysis 17 Type and crossmatch may be considered in patients who are vulnerable to complications of bleeding. A creatinine value or estimated glomerular filtration rate should be obtained prior to angiography; the normal range varies according to patient size, lean body weight, and local reference values. An abnormal value does not necessarily preclude intervention, but renal protective measures such as pre- and postprocedural hydration, minimization of contrast load, the use of alternative contrast agents such as CO2, and adjunctive use of intravascular ultrasound (IVUS) should be entertained in patients with renal compromise who are not already on dialysis.
The selection of an appropriate access site is an important factor in procedural success as well as both patient and operator comfort, and should be determined beforehand to guide room setup and patient positioning. Ideally, the access into the venous system should allow treatment of the entire thrombus burden. This is often possible by using a combination of both anterograde and retrograde approaches, dependent on DVT location and extension. Peripheral access (using the veins of the calf or forearm) is desirable. It is relatively easy to perform with ultrasound, due to the superficial location of the structures, and safe, since the veins can be easily compressed upon procedure completion. Unfortunately, peripheral veins are relatively small, which can make cannulation difficult and limit the size of devices that can be safely delivered. Superficial veins are prone to spasm, complicating access and occasionally preventing advancement of devices through these vessels, even over a wire. The popliteal and brachial veins are larger and can accommodate the majority of devices and sheaths required for most procedures. Accessing these vessels may require puncture of a thrombosed segment, as the DVT may extend across these segments. This approach via a thrombosed vein may leave portions of the DVT untreated; however, by establishing flow central to the thrombosis, symptomatic improvement is often accomplished. For popliteal vein access, prone or “frog-leg” positioning is required. The advantage of the latter is that this allows access to the jugular veins without repositioning, if necessary, and is generally better tolerated by the patient for longer procedures. Common femoral vein access can be used in the treatment of iliofemoral, iliocaval, central thoracic, jugular, or upper extremity DVT; rarely, it can also be applied for both contralateral (“up-and-over”) or ipsilateral (anterograde) lower extremity treatment. These veins are large and generally quite forgiving, and this approach is familiar to interventional staff and relatively conducive to most room setups. Central greater saphenous vein access is an option in most cases where common femoral access is applicable, and has several advantages: if damaged or thrombosed, the consequences to the patient are often less severe, there is less chance of missing a segment of disease within the common femoral vein that could compromise inflow when treating iliofemoral disease, and the superficial venous system is generally exposed to less pressure and therefore it is easier to obtain hemostasis at the termination of the procedure. Internal or even external jugular venous access can be utilized for therapies involving all four extremities as well as for caval thrombosis, and is frequently combined with more peripheral accesses for more complex procedures, particularly when some degree of chronic occlusion is also present. With all access points, it is important to note that approaching a limb vein from a central location may require navigation of valves in order to pass a wire, whereas a peripheral approach mitigates this issue.
The most common thrombolytic agent currently used in the United States is alteplase, which is a recombinant form of human tissue plasminogen activator (tPA). Other modified recombinant agents used throughout the world include reteplase and tenecteplase. Historically, streptokinase and urokinase were also used for thrombolysis; however, these agents are now used infrequently. The concentration of tPA used depends on the context of the intervention, ranging from high concentrations for pulse-spray thrombectomy to more dilute preparations used for overnight pharmacologic thrombolysis.
Equipment that may be helpful throughout the procedure should ideally be gathered prior to beginning the intervention. Devices that may be utilized include a variety of sheaths, directional and balloon catheters, wires (both hydrophilic and standard, including exchange lengths), infusion pumps, an ultrasound-enhanced thrombolysis unit, thrombolysis infusion catheters, rheolytic thrombectomy, IVUS catheters and the IVUS display unit, and any of a number of mechanical or pharmacomechanical devices and their associated catheters.
4.3.3 Technical Tips and Tricks and Procedural Details
Once an access site has been selected, ultrasound-guided access is favored to decrease the risk of bleeding, particularly if thrombolysis is planned. Micropuncture access using a 21-gauge needle is favored over 18-gauge needle access, with single-wall technique preferred by the authors. If a peripheral access vessel was selected, initial venography can be performed through the micropuncture dilator for an initial assessment of thrombus burden, flow characteristics, and variant anatomy. As with any interventional procedure, initial sheath size is selected based on the required diameter for insertion of the instruments planned for intervention; sheath length is chosen according to the depth of the access vessel and the distance to the area of intervention from the venotomy site. Ideally, the sheath tip should terminate relatively close to the area of intervention to facilitate intermittent venography via the sheath after interventions. Anticoagulation should be continued throughout and following the procedure.
Often, a Bentson wire or J-tipped wire will easily traverse acute and subacute thrombus (the “wire test”). A standard angled-tip hydrophilic wire can be used to navigate through firm organized thrombus, chronic occlusions, and venous stenoses when standard wires fail to pass. A 4- or 5-French end-hole catheter with an angled tip can help support the wire for increased pushability. After removing the wire, an injection of contrast can be performed through the catheter as it is pulled back to define the full extent of DVT once it has been traversed. The procedure then varies depending on the chosen method of thrombus removal.
For pharmacologic thrombolysis, the length of the thrombus must be measured to select an infusion catheter with an appropriate treatment length. A standard or ultrasound-enhanced infusion catheter approximating the length of the DVT is placed across the thrombosed segment, attempting to place as many side holes as possible within the thrombus so thrombolytic is not injected directly into the systemic circulation. It is important to have the most proximal and distal side holes within a patent vessel, if possible, to treat the ends of the thrombus. CDT is then initiated; progress must be monitored no less frequently than every 24 hours by performing a “lysis check” venogram. Typical tPA infusion rates range from 0.5 to 1.0 mg/h, and usually a fixed rate of heparinized saline (~300–500 units/h) is administered through the side-port of the access site sheath to prevent sheath thrombosis. Many operators elect to maintain low-dose, subtherapeutic heparin infusion without adjusting according to PTT nomograms, due to concerns for increased risk of bleeding with therapeutic heparinization. However, there is a trend toward utilizing therapeutic heparinization during thrombolysis, and there is no definitive consensus in this regard. Close clinical follow-up during the infusion is imperative. At most institutions, patients undergoing thrombolytic therapy require admission to an intensive care or step-down unit familiar with management of patients undergoing thrombolysis. This is necessary predominately to monitor for signs of access site or remote bleeding, as well as to closely monitor progress in the affected limb. Many institutions vary in their practice of following serum fibrinogen values and blood counts during lysis, and this is discussed in detail below.
Mechanical thrombectomy comprises a broad range of techniques utilized to disrupt, dislodge, and remove thrombus from the vein. Inflation of a noncompliant balloon within a segment of thrombus can help macerate the thrombus, which is occasionally performed prior to thrombolysis to help expose an increased amount of thrombus surface area to the thrombolytic agent. High-pressure angioplasty is generally not necessary for this purpose, although not uncommonly one or more venous stenoses are encountered. If visualized, this stenosis can be angioplastied early in the procedure to encourage outflow following debulking of the clot. Passage of a nominally inflated balloon sized 1:1 to the vessel in question in a peripheral-to-central direction can also be used to displace thrombus into the central veins, as with arteriovenous fistula thrombectomy. However, caution should be taken when treating a large thrombus burden as this clot embolizes to the lungs.
A multitude of mechanical thrombectomy devices currently exist on the market (over 30 as of 2016), a full discussion of which is beyond the scope of this chapter. The prototypical device in this class is the Arrow-Trerotola Percutaneous Thrombolytic Device (Teleflex, Wayne PA), which is commercially available in both over-the-wire (OTW) and non-OTW varieties. Both device types are available in 65 and 120 cm lengths. The device is unsheathed within the thrombus and activated, causing rapid rotation of the fragmentation basket and maceration of the thrombus. Many other currently available devices are a variation on this theme. The major limitation in these devices is the lack of thrombus removal and the potential for clinically significant distal embolization. In general, the authors reserve the use of these devices for tough, refractory thrombus, particularly material adherent to the vessel wall that is unsuccessfully treated using other methods.
The AngioJet peripheral thrombectomy system (Boston Scientific, Marlborough, MA) is a mechanical rheolytic thrombectomy device that can be utilized to both disrupt and remove acute and subacute thrombus from the vessel. The device is advanced over a guidewire through the thrombus and can be used in two modes: pulse-spray mode, which administers rapid bursts of a user-defined solution into the vein without aspirating, and thrombectomy mode, which simultaneously infuses and aspirates. In pulse-spray mode, typically a 50- to 100-mL solution of 25 to 50% tPA is administered into the thrombus by slowly advancing it over the wire while the device is activated to lace the thrombus with tPA. The medication is typically allowed to dwell for 10 to 30 minutes. This administration facilitates softening and partial dissolution of the thrombus, which can then be removed by using the thrombectomy mode. If the patient is at a high risk of bleeding, pulse-spray can also be performed with saline. A variety of catheter diameters and lengths are available, ranging from 3 to 8 French and from 50 to 150 cm. Each catheter is connected to the same base unit that drives infusion and aspiration. Contrast can be injected through a side-port intermittently to monitor treatment progress. In using the Angiojet, systemic heparinization is recommended after gaining venous access in order to prevent acute rethrombosis. Up to 60 mL/min can be aspirated using this device, and therefore it should be used sparingly in flowing blood. Transient hemolysis can occur during treatment, and therefore the device should not be used for more than 480 seconds of total treatment time (or 240 seconds in flowing blood) due to risk of renal injury. Patients and caregivers should be warned that most patients will experience some degree of hemoglobinuria following the use of this device, and hydration afterward is recommended. This system is far more effective when treating acute thrombus and is relatively limited for the treatment of organized, chronic DVT.
Peripheral suction thrombectomy using the Indigo device (Penumbra, Alameda, CA) is a relatively recent addition to the thrombectomy armamentarium. The system consists of 4.1- to 8-French suction catheters (ranging in length from 85 to 115 cm), a catheter-specific wire-mounted separator device, a collection canister, and the MAX pump unit. The catheters are made of a reinforced material that prevents collapse during suction, and the catheter tips are available in straight and angled varieties. The catheter is advanced over a wire through the hemostatic valve of the sheath and positioned in the area of the thrombus. The wire is removed and the separator wire is advanced through a rotating hemostatic valve attached to the back end of the catheter. The suction tubing is connected to the side-port of the rotating hemostatic valve. The catheter is manipulated through the thrombus while suction is applied. The operator intermittently pulls the separator device back into the catheter to clear thrombus adhered to the catheter tip. Close attention must be paid to the rate of aspirate obtained in the collection canister, as a large amount of blood can be removed when the thrombus has cleared and blood flow is restored. For this reason, the authors recommend a type and screen prior to use of this device, as well as a hemoglobin and hematocrit level before and after the procedure. Similar to the AngioJet, this device is much more useful for acute and subacute thrombus, and its usefulness for chronic DVT is relatively limited.
In some settings, a large volume of thrombus may be present in a larger vessel that does not cause complete occlusion (e.g., free-floating thrombus extending above an IVC filter). In these settings, thrombectomy can be risky because of the potential for a relatively large amount of blood loss, and the risk of embolizing substantial thrombus to the lungs. The AngioVac venous drainage system (AngioDynamics, Latham, NY) is an excellent option in these situations, as the material removed is passed through a filter to trap solid material such as thrombus, with the remaining blood products returned to the patient through an extracorporeal bypass circuit. The AngioVac circuit can be connected to any centrifugal bypass pump console, but requires a perfusionist to monitor the extracorporeal bypass process. The 22-French, coil-reinforced cannula has a tip that assumes a funnel-shaped configuration once the balloon is inflated, allowing it to fill the cross-sectional area of the vessel and decrease the likelihood of embolization past the catheter tip. The device is highly efficient and can remove large amounts of thrombus en bloc, without the fear of massive blood loss. Catheter clogging is rare. Potential limitations include the need for an available perfusionist, the large access sheath required for catheter delivery, and the need for an additional venous access site for the return of blood products to the patient.
Regardless of the device used, once the thrombus has been treated, venography should be performed in order to monitor treatment progress and evaluate for underlying venous stenosis, which may have predisposed the patient to the thrombosis. Adjunctive treatments may include balloon angioplasty and/or stenting. Following treatment, therapeutic anticoagulation should be initiated for all patients in whom it is safe.
The use of caval filtration during DVT treatment is controversial. Clinically significant PE has been reported as high as 5% during catheter-directed DVT therapy, with up to 10% of in-hospital deaths following treatment attributed to PE. 18 In one recent study, more than 40% of patients that underwent prophylactic IVC filter placement prior to aspiration thrombectomy demonstrated thrombotic material within the filter during the caval venogram prior to filter retrieval. 19 Until further evidence is compiled, the placement of a caval filter is at the discretion of the operator. The decision should take into account the respiratory status of the patient, preexisting PE, the location and volume of thrombus being treated, and the specific method of therapy. At the authors’ institution, IVC filters are used sparingly during DVT interventions, primarily in the setting of large-volume iliocaval thrombus. Superior vena cava (SVC) filter placement is far less common than IVC filters; the authors feel that filters are generally unnecessary during DVT interventions above the heart. 20
4.3.4 Potential Complications or Pitfalls
Significant complications of DVT intervention are rare. During thrombolysis, the most common and potentially most severe complications are related to bleeding. Minor bleeding complications, such as oozing at the puncture site or local hematoma formation, are around twice as common in patients undergoing thrombolysis than in those receiving systemic anticoagulation alone, with a relative risk of 2.2. 21 Access site bleeding should initially be managed using conservative measures: application of pressure to the site of bleeding and/or the use of hemostatic adjuncts such as hemostatic pads or powder.
Close hemodynamic monitoring and frequent measurement of blood counts is suggested during thrombolytic therapy, to aid in early identification of bleeding in nonvisible locations, such as the retroperitoneum. Early identification of bleeding also guides the need to cease therapy or search for an obscure site of bleeding with additional imaging. Routine serum fibrinogen value monitoring is controversial; however, most investigators still report its use as a tool to alert clinicians of a potential increase in the risk of bleeding complications. There are conflicting data regarding the specific level of serum fibrinogen below which bleeding risk becomes prohibitive. 22 , 23 Rapid decreases in fibrinogen level (e.g., >50% decrease from baseline) may prompt a decrease in the dose of tPA administration, more frequent fibrinogen monitoring, and the consideration of a venographic assessment earlier than originally planned. Fibrinogen levels below 100 mg/dL should prompt the consideration of temporary or complete cessation of therapy, and potentially the administration of cryoprecipitate. Aggressive resuscitative measures should be initiated in patients with signs of significant remote bleeding, including the administration of packed red blood cells, platelets, and plasma, as appropriate.
Intracranial bleeding has been reported in up to 2.5% of patients undergoing thrombolysis, and can be potentially devastating. Any new focal neurologic signs should be considered an intracranial bleed until proven otherwise; immediate interruption in thrombolysis and a noncontrast CT of the head are prudent. PE is another concern for patients undergoing treatment of DVT, particularly those with iliocaval thrombus. No statistically significant increase in PE has been reported for patients undergoing thrombolysis alone, but up to 2.5% of patients undergoing pharmacomechanical thrombectomy may develop clinically significant PE.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree