4 Intracranial Aneurysms
4.1 Imaging of Unruptured Saccular Aneurysms
4.1.2 Clinical Case
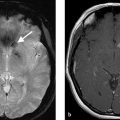
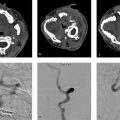
A 73-year-old female with severe frontal headache and cognitive decline (Fig. 4.1).
4.1.2 Description of Imaging Findings and Diagnosis
Diagnosis
Giant partially thrombosed aneurysm with associated perianeurysmal edema. Digital subtraction angiography (DSA) demonstrates a large luminal component but there is an obvious mismatch between the size of the aneurysm on DSA and the size on MRI.
4.1.3 Background
Saccular cerebral aneurysms comprise roughly 90% of all cerebral aneurysms and are the most common cause of nontraumatic subarachnoid hemorrhage. The prevalence of saccular aneurysms in the general population ranges from 2 to 8% depending on the region (higher prevalence in Japan and Finland). Two-thirds of aneurysm patients are women. Among patients with intracranial aneurysms, 10–20% have multiple lesions. Saccular aneurysms are associated with a variety of abnormal connective tissue diseases; however, most aneurysms are sporadic. Patients with connective tissue disease or a family history of intracranial aneurysms will often undergo screening for intracranial aneurysms even when asymptomatic.
Saccular aneurysms are histologically characterized by disruption of the internal elastic lamina at the entrance site/neck of the aneurysm. The histopathologic features of the intima, media, and adventitia are the prime determinants of aneurysm natural history. Unruptured aneurysms are characterized by an intact endothelium and smooth muscle layer with the absence of inflammatory cells in the vessel wall. In contradistinction, ruptured and unstable aneurysms are characterized by endothelial disruption, absence of smooth muscle cells, and inflammatory cell infiltration into the media and adventitia. Adventitial inflammation results in media thinning, promoting aneurysm formation. Histopathologic studies have demonstrated that unstable aneurysms are also characterized by wall thinning, organizing thrombus, and the increased expression of inflammatory markers.
Management of unruptured aneurysms (i.e., to treat or not to treat) is one of the major dilemmas in cerebrovascular medicine. In the United States, for example, roughly 30,000 patients/year suffer from an aneurysmal SAH whereas somewhere between 5 and 10 million people have an unruptured aneurysm. A number of decision aids have been published to guide in the management of these lesions (Table 4.1). However, the primacy of imaging findings in guiding treatment decisions remains undisputed.
4.1.4 Imaging Findings
The role of the diagnostic neuroradiologist in evaluation of a suspected aneurysm patient is to (1) detect the aneurysm, (2) characterize the aneurysm, and (3) determine the effect the aneurysm is having on the surrounding nonvascular structures.
Both CT angiography and MR angiography are excellent imaging modalities for the detection of cerebral aneurysms. A number of modern series have found that multi-detector row CTA and the time of flight MRA have sensitivities and specificities of greater than 90% in the detection of cerebral aneurysms measuring 3 mm or larger. When evaluating these angiographic images, there are a few key principles to keep in mind to increase one’s ability to detect a cerebral aneurysm. First, one must evaluate both the thin-cut source images as well as the thick-cut maximum intensity projection images. MIP images allow most aneurysms to “pop-out,” but occasionally one can be tricked into thinking that a prominent vascular loop is an aneurysm if thin-cut images are not used to verify the finding on the MIPs. Thin-cut images also allow for the detection of smaller aneurysms, which are concealed on the maximum intensity projections (MIPs). Thin slices are also useful in evaluating aneurysms of the cavernous sinus/carotid siphon, especially on CTA. Second, it is important to review all multiplanar reformatted images as some aneurysms are more obvious when viewed in the coronal and sagittal planes. Third, a 3D representation of the circle of Willis is invaluable for aneurysm detection. Each bifurcation point should be closely inspected and each potential finding should be verified onsource images. Whenever a patient has one aneurysm, always look for more. The most common site to find a second aneurysm is in the same location as the first aneurysm, but on the contralateral side (i.e., mirror image aneurysms) (Fig. 4.2).
Characterization of the aneurysm is essential to determining treatment options. Measurements should be obtained of the aneurysm neck, aneurysm height, and aneurysm width. The maximum neck width of the aneurysm is important in treatment planning as wide necked aneurysms often require stent-assisted coiling, flow diversion, or surgical clipping, whereas narrow-necked aneurysms can be treated with simple endovascular coiling. The shape of the aneurysm should be described as smooth (i.e., no surface irregularity, well rounded), lobulated (i.e., two more or less equally sized lobules), or having a daughter sac (i.e., a small outpouching arising from the aneurysm sac). Saccular and lobulated aneurysms have been shown to have more favorable natural histories than those which have daughter sacs (Fig. 4.3).
The orientation of the aneurysm is also important (inferior, superior, posterior, anterior, etc.). For example, an Acom aneurysm that points superiorly or posteriorly is favorable for endovascular management due to the presence of perforators draped over the aneurysm sac, whereas one directed inferiorly is favorable for surgical clipping as it is difficult to get a good working projection for endovascular treatment. The relationship of the aneurysm with branch vessels (i.e., is the vessel coming from the aneurysm neck) and smaller perforators in the vicinity of the aneurysm (i.e., thalamoperforators, heubner, anterior temporal artery, lenticulostriates, etc.) is essential. Anatomic variants (i.e., hypoplastic or absence Pcom or A1, fenestration, MCA trifurcation vs. bifurcation, etc.) can also affect treatment decisions.
The relationship between the aneurysm and adjacent nonvascular structures is something that is often overlooked when evaluating an intracranial aneurysm patient. For aneurysms located along the cavernous ICA, careful attention should be paid to the relationship between the aneurysm and the sphenoid sinus as large or giant cavernous carotid aneurysms can erode in the sinus and result in life-threatening epistaxis (Fig. 4.4). For ICA aneurysms, the relationship between the aneurysm and the clinoid process is helpful in determining if a lesion is intradural or extradural as those below the anterior clinoid process are considered extradural (and not at risk of SAH), whereas those above are intradural (and at risk of SAH). For those at are paraclinoid, some are intradural and some are extradural. The relationship between aneurysms and the cranial nerves needs to be commented on and can best be evaluated on high resolution fast imaging employing steady-state acquisition (FIESTA) imaging (Fig. 4.5). Paraophthalmic and Acom/ACA aneurysms can exert substantial mass effect on the optic nerve. Pcom, basilar tip, and PCA aneurysms can cause mass effect on the oculomotor nerve. The relationship between the aneurysm and the adjacent cerebral parenchyma is also important. Large and giant aneurysms can sometimes exert substantial mass effect on the adjacent brain parenchyma and result in edema and neurological deficit even in the absence of rupture (Fig. 4.1 , Fig. 4.2). This is especially true for giant thrombosed aneurysms, which are sometimes mistaken for large extra-axial tumors.




4.1.5 What the Clinician Needs to Know
Anatomic characteristics of the aneurysm including size, neck width, location, orientation, and relationship to branch vessels.
Morphology of the aneurysm including presence of lobulations or daughter sac.
Aneurysm multiplicity.
Relationship between the aneurysm and adjacent nonvascular tissues including osseous structures, cranial nerves, and brain parenchyma.
4.1.6 High-Yield Facts
Saccular aneurysm rupture risk is dictated primarily by size (large size → more rupture) and location (i.e., Acom/ACA and posterior circulation aneurysms at higher risk of rupture).
The term giant aneurysm is typically reserved for aneurysms which are 25 mm or larger. These lesions often have intraluminal thrombus and can exert mass effect on surrounding structures.
Mirror image aneurysms are the most common location for patients with multiple aneurysms.
Further Reading
[1] HaiFeng L, YongSheng X, YangQin X, et al. Diagnostic value of 3D time-of-flight magnetic resonance angiography for detecting intracranial aneurysm: a meta-analysis. Neuroradiology. 2017; 59(11):1083–1092 [2] Turan N, Heider RA, Roy AK, et al. Current Perspectives in Imaging Modalities for the Assessment of Unruptured Intracranial Aneurysms: A Comparative Analysis and Review.World Neurosurg. 2018; 113:280–292 [3] Krings T, Piske RL, Lasjaunias PL. Intracranial arterial aneurysm vasculopathies: targeting the outer vessel wall. Neuroradiology. 2005; 47 (12):931–9374.2 Imaging of Ruptured Aneurysms
4.2.1 Clinical Case
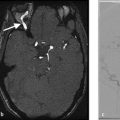
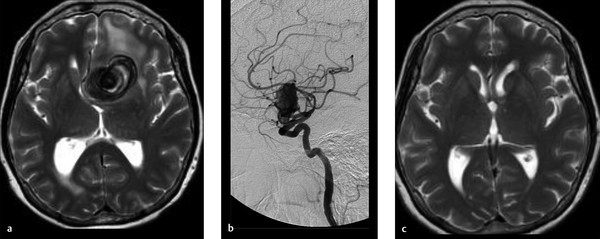
A 73-year-old female with sudden onset severe headache followed by decreased level of consciousness. Current GCS is 11 (Fig. 4.6).
4.2.2 Description of Imaging Findings and Diagnosis
Diagnosis
Intraparenchymal hemorrhage in the bilateral frontal lobes, greater on the right, with associated diffuse SAH. CTA demonstrates a small pericallosal aneurysm pointing toward the right.
4.2.3 Background
Saccular cerebral aneurysms are the most common cause of nontraumatic SAH. Rupture of a cerebral aneurysm results in the extravasation of blood into the subarachnoid space, which triggers a cascade of events that can result in severe disability or death. Failure to diagnose and treat an aneurysmal SAH can result in further impairment due to aneurysm rebleeding and delayed cerebral ischemia from cerebral vasospasm. Although lumbar puncture is the gold standard for the diagnosis of SAH, CT has emerged as the diagnostic modality of choice with sensitivity and specificity of over 99% in the first days after the SAH.
The diagnostic neuroradiologist’s job does not stop at the identification of the SAH and the culprit aneurysm. Rather, the neuroradiologists is responsible for obtaining and relaying all the information that is required to make a determination regarding the ideal management strategy for the aneurysm; namely, factors that will determine whether the aneurysm will be clipped or coiled.
4.2.4 Imaging Findings
An imaging checklist in the evaluation of ruptured aneurysms is provided in Table 4.2. The first task of the neuroradiologist is to properly localize the aneurysm. At most institutions, CT angiography is the imaging modality of choice and is performed prior to conventional angiography. The use of thin-slice multidetector row CTA with MIP and 3D reconstructions allows for a 90% sensitivity and specificity for detection of intracranial aneurysms. One clue regarding the location of the aneurysm is identifying the region of the densest hematoma. For example, dense subarachnoid blood in the left sylvian fissure should prompt one to closely inspect the left MCA bifurcation while dense blood in the interhemispheric fissure should prompt one to closely examine that ACA/Acom complex. Whenever a patient has one aneurysm, always look for more. The most common site to find a second aneurysm is in the same location as the first aneurysm, but on the contralateral side (i.e., mirror image aneurysms). A good rule of thumb is that posterior circulation aneurysms and aneurysms along the ICA typically go to coiling while those of the MCA go to clipping.
Neuroradiologists need to determine and characterize in the aneurysm configuration and geometry. Measurements of the height, width, and neck width are needed for treatment planning. Aneurysms which are narrow-necked are more amenable to simple coiling while wider neck aneurysms will require coiling with adjuvant techniques or clipping (Fig. 4.7).
Two factors that are commonly underreported in diagnostic neuroradiology reports include the projection of the aneurysm and the proximity of large vessels or perforators to the aneurysm neck and dome. For example, at the Acom complex, aneurysms which project inferiorly are typically reserved for clipping as it is difficult to get a working projection on biplane; while, aneurysms which project superiorly are reserved for coiling due to the presence of perforators draping over the aneurysm itself (Fig. 4.8). Regarding the presence of nearby vessels, any time perforators are surrounding the aneurysm neck or dome, coiling is preferred. Meanwhile, whenever there is a vessel coming from the aneurysm neck, clipping is preferred. Any calcifications at the aneurysm neck need to be identified as these can complicate clipping procedures and may sway the treatment decision toward coiling (Fig. 4.9).
In addition to aneurysm characterization, assessment of the cerebrovascular tree for other aneurysms, anatomical variations (i.e., hypoplastic vessels, fenestrations, fetal PCA, etc.), atherosclerosis, and elongative arteriopathies is important for treatment planning.
Lastly, roughly 10–20% of ruptured aneurysm patients have multiple intracranial aneurysms. Unless these aneurysms are in a single operative field or operative bed, most practitioners hesitate to treat multiple aneurysms in a single operation, even in the setting of a SAH (Fig. 4.10). Thus, it is important to be able to determine, with a reasonable level of confidence, which aneurysm is the culprit aneurysm. Blood distribution is the first important hint (Table 4.3). Regarding aneurysm characteristics, factors such as irregular morphology, larger size, presence of a bleb or daughter sac, local vasospasm, and a high aspect ratio (i.e., height to neck width) are imaging findings which suggest that an may be the culprit aneurysm in setting of SAH and multiple aneurysms.
Non-Contrast CT Findings | Aneurysm Characteristics |
SAH Extent | Location |
SAH Distribution | Size |
Large Clot/Parenchymal Hemorrhage | Multiplicity |
Intraventricular Hemorrhage | Neck Size and Dome-to-Neck Ratio |
Hydrocephalus | Irregular Morphology |
Calcified Neck or Dome | |
Anatomic Characteristics | |
Proximity to perforator vessels | Aneurysm Etiology |
Vessels coming from neck | Saccular |
Elongative arteriopathy | Dissecting |
Anatomic Variants | Partially thrombosed |
Atheroscleroisis | Traumatic |
Local Vasospasm | Mycotic |




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree