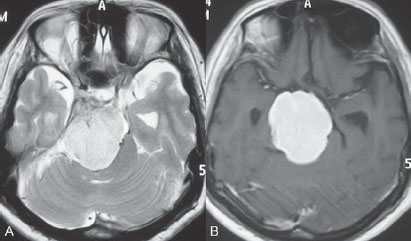
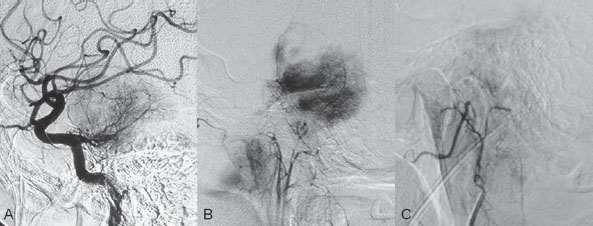
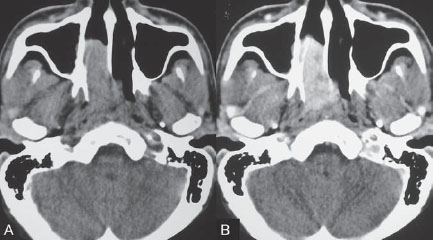
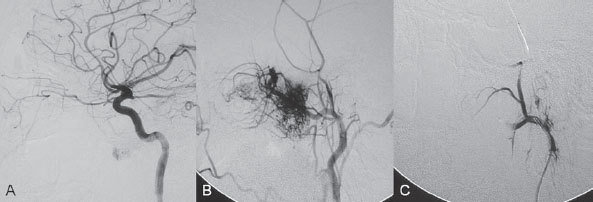
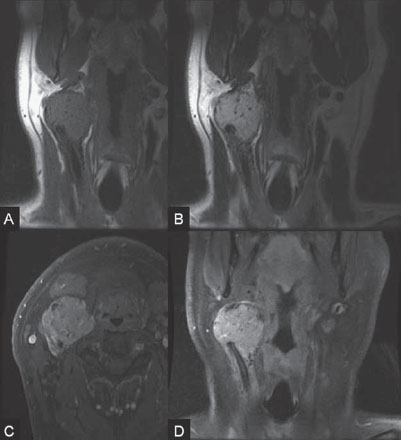
PART VTumors A 50-year-old woman has a history of headaches for nearly a year. One month before admission, she developed numbness of the right side of her face with ataxia. Physical examination shows decreased sensation in the territory of cranial nerve V, mild right-sided facial weakness, and ataxia. No motor weakness is present. MRI is performed, followed by angiography with preoperative embolization. Fig. 36.1 MRI. (A) T2-weighted and (B) post-gadolinium T1-weighted images. MRI MRI revealed a well-defined extra-axial mass lesion at the right petroclival region with hypointense T1/hyperintense T2 signal and intense homogeneous enhancement on a post-contrast study. The lesion caused a significant mass effect on the right side of the pons and midbrain (Fig. 36.1). A linear, enhancing dural tail was observed along the clivus. The findings were consistent with the diagnosis of a petroclival meningioma. DSA The study demonstrated a hypervascular tumor blush, supplied predominantly by branches of the right meningohypophyseal trunk from the right internal carotid artery (ICA) and meningeal branches from the right neuromeningeal trunk of the right ascending pharyngeal artery (Fig. 36.2 A,B). Minimal supply from branches of the left meningohypophyseal trunk and left ascending pharyngeal artery was also observed. Fig. 36.2 DSA. (A) Right ICA and (B) right ascending pharyngeal artery angiograms in lateral view demonstrate a hypervascular tumor blush. (C) Angiogram of the right ascending pharyngeal artery after particle embolization shows obliteration of the supply to the tumor. Right petroclival meningioma EQUIPMENT DESCRIPTION Following diagnostic angiography, a 5F multipurpose catheter was advanced into the proximal right external carotid artery (ECA). An over-the-wire microcatheter was introduced over a micro-guidewire into the neuromeningeal trunk of the right ascending pharyngeal artery. A mixture of 300- to 500- μm polyvinyl alcohol particles with contrast media was injected slowly under a blank roadmap until stagnation of the distal branches was observed. The microcatheter was removed, and the 5F multipurpose catheter was advanced into the proximal left ECA. The microcatheter was then placed into the left ascending pharyngeal artery, followed by particle embolization with the same technique. The microcatheter was removed, and the final control run showed obliteration of the external carotid supply to the tumor (Fig. 36.2C). Attempts to catheterize the meningohypophyseal trunks from the ICAs failed to reach a safe position for particle or glue embolization. The patient underwent surgical removal of the tumor within 24 hours after the procedure without any complications. Meningiomas are extra-axial tumors arising from arachnoid cells of the meninges, which originate from the neural crest. They account for ~13 to 20% of all primary intracranial tumors and can be found at any location along the arachnoid membrane, most commonly along the cerebral convexity. The peak incidence is at 45 years of age, with a strong female predominance. Malignant lesions are very rare, but metastases may occur by seeding via the cerebrospinal fluid. Extraneural metastases to the lungs, liver, and lymph nodes may also occur. Because most meningiomas are benign and curable tumors, complete surgical resection remains the treatment of choice. CT MRI CTA/MRA RADIOSURGERY SURGICAL TREATMENT ENDOVASCULAR TREATMENT Published Literature on Treatment Options Many studies in previous literature have shown benefits of the preoperative embolization of meningiomas, especially larger ones, to help reduce blood loss during surgery, time required for surgical resection, and length of hospital stay. Blood loss is significantly reduced if the tumor is devascularized more than 90%. In the past, it was generally accepted that the optimal timing for surgery after embolization to reduce the blood loss was within 24 hours; however, this concept has changed in the past years. Several authors have used permanent liquid embolic agents (glue and Onyx), which makes it possible to delay surgery until maximum tumor necrosis and softening occur. This phenomenon also occurs with the use of smaller particles, which penetrate and occlude the capillary bed of the tumor; when these embolic agents are used, the optimal interval between embolization and surgery for tumor softening is 7 to 9 days. The complication rates for the preoperative embolization of meningiomas vary among institutions but are generally accepted to be between 5 and 7%. A significant risk factor for complications is the use of smaller particles or liquid embolic agents. The use of a temporary balloon has been advocated to reduce the chance of stroke during the embolization of branches of the ICAs and VAs. The balloon can be positioned across the origin of the feeding dural vessel after it has been superselectively catheterized. The balloon is inflated while the embolization is performed via the microcatheter with either particles or liquids. Another method is to position the balloon distal to the tumor-feeding dural branch and inflate the balloon while embolization is performed in the main parent vessel ((ICA, VA) through a microcatheter positioned immediately adjacent to the origin of the tumor-feeding dural branch. Flow into the dural branch must be significant for the embolic material to reach this vessel while the balloon is inflated for distal protection. Once flow to the tumor has greatly diminished, the parent vessel is “cleaned” with repeated injections of saline and either removal of the mixture of blood and saline or intentional flushing of the column down to the external carotid system. The balloon system is then deflated and removed. Obviously, anticoagulation is necessary under these circumstances PEARLS AND PITFALLS__________________________________________________ Dowd CF, Halbach VV, Higashida RT. Meningiomas: the role of preoperative angiography and embolization. Neurosurg Focus 2003;15(1):E10 Engelhard HH. Progress in the diagnosis and treatment of patients with meningiomas. Part I: diagnostic imaging, preoperative embolization. Surg Neurol 2001;55(2):89–101 Lasjaunias P, Berenstein A, terBrugge K. Surgical Neuroangiography. Vol 2: Clinical and Endovascular Treatment Aspects in Adults. 2nd ed. New York, NY: Springer-Verlag; 2004 Tymianski M, Willinsky RA, Tator CH, Mikulis D, TerBrugge KG, Markson L. Embolization with temporary balloon occlusion of the internal carotid artery and in vivo proton spectroscopy improves radical removal of petrous-tentorial meningioma. Neurosurgery 1994;35(5):974–977, discussion An 18-year-old man presents with a history of recurrent episodes of epistaxis for 3 months and progressive stuffiness of the right nostril. His neurologic examination is normal. CT is performed, followed by angiography with preoperative embolization. Fig. 37.1 (A) Unenhanced and (B) post-contrast axial CT scans at the level of the nasopharynx. CT CT showed a slightly hyperdense mass at the right posterior nasal cavity involving the nasal septum and extending into the nasopharynx. Marked, slightly heterogeneous enhancement was seen on a post-contrast study (Fig. 37.1). Given the age and sex of the patient, these findings led to the diagnosis of a juvenile angiofibroma. DSA The study revealed a hypervascular tumor blush, supplied predominantly by branches of the right internal maxillary artery and ascending palatine artery from the right facial artery (Fig. 37.2 A,B). Minimal supply from the mandibular artery of the right internal carotid artery (ICA) through the foramen lacerum was also observed. Right-sided juvenile angiofibroma Fig. 37.2 DSA. (A) Right ICA and (B) right internal maxillary artery angiograms in lateral view. (C) Post-particle embolization angiogram of the right internal maxillary artery shows obliteration of the supply to the tumor. EQUIPMENT DESCRIPTION Following diagnostic angiography, a 5F multipurpose catheter was advanced into the proximal right external carotid artery. An over-the-wire microcatheter was introduced over a micro-guidewire into the distal internal maxillary artery beyond the origin of the middle meningeal artery. A mixture of 355- to 500-μm polyvinyl alcohol particles with contrast media was injected slowly under a blank roadmap until stagnation of the distal branches was observed. The microcatheter was then advanced into the right ascending palatine artery of the right facial artery, followed by particle embolization with the same technique. The microcatheter was removed, and the final control run showed obliteration of the external carotid supply to the tumor (Fig. 37.2C). The patient underwent further surgical removal of the tumor within 24 hours after the procedure without any complications. Juvenile angiofibromas are relatively uncommon tumors of vascular origin that are histologically benign but locally invasive. They account for ~0.5% of all head and neck tumors, typically developing in male adolescents. The peak age is ~14 to 17 years. The clinical symptoms of a juvenile angiofibroma are related directly to its size and extension. The most common are nasal obstruction and epistaxis. The tumor typically originates in the posterior nasal cavity and usually extends to the pterygopalatine fossa. Occasionally, it may arise from the nasal septum. The classification system of Fisch is often employed for staging juvenile angiofibromas; stages I and II tumors are still localized to the nasal cavity, nasopharynx, and pterygopalatine fossa, whereas stages III and IV tumors involve the infratemporal fossa, orbit, and skull base. Complete surgical resection is the best treatment choice; however, local recurrence may be seen in up to 35% of cases. Malignant transformation is extremely rare and in most cases reported in the literature is secondary to radiation therapy. CT MRI CTA/MRA RADIOSURGERY SURGICAL TREATMENT Published Literature on Treatment Options Preoperative embolization is currently generally accepted as the standard management of juvenile angiofibromas, particularly large tumors with skull base extension (Fisch stages III and IV). In smaller tumors (stages I and II), several studies have shown that preoperative embolization helps to decrease blood loss during endoscopic surgery, the duration of packing, and length of hospital stay. Although several reports have been published about the direct percutaneous puncture and intratumoral embolization of juvenile angiofibromas, this technique requires significant operator experience to avoid the potentially devastating complications of ICA stroke. PEARLS AND PITFALLS__________________________________________________ Andrade NA, Pinto JA, Nobrega MdeO, Aguiar JE, Aguiar TF, Vinhaes ES. Exclusively endoscopic surgery for juvenile nasopharyngeal angiofibroma. Otolaryngol Head Neck Surg 2007;137(3):492–496 Casasco A, Houdart E, Biondi A, et al. Major complications of percutaneous embolization of skull-base tumors. AJNR Am J Neuroradiol 1999;20(1):179–181 Valavanis A, Christoforidis G. Applications of interventional neuroradiology in the head and neck. Semin Roentgenol 2000;35(1):72–83 A 42-year-old man feels a slowly growing mass in his neck below the angle of the mandible. On examination, a nontender mass is palpated in the carotid space that is mobile in the lateral plane but immobile in the craniocaudal plane. There are no signs of pain or dysphagia, and the patient does not experience syncope. Contrast-enhanced MRI is performed. Fig. 38.1 MRI of the neck. (A) T1 pre-contrast, (B) T1 post-contrast, (C) axial fat-suppressed T2, and (D) coronal T1 post-contrast fat-suppressed sequences demonstrate an intensely enhancing T2-hyperintense lesion with intralesional flow voids at the carotid bifurcation. This lesion separates the ECA and ICA. MRI MRI demonstrated a densely enhancing T2-hyperintense lesion in direct proximity to the right neurovascular bundle with intratumoral flow voids and separation of the external and internal carotid arteries (Fig. 38.1). No other tumors were visible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree