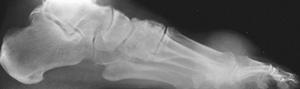
FIGURE 5-54 Gouty arthritis of the first MTP joint. A) Extensive erosive changes of the medial aspect of the first MTP joint, primarily involving the head of the metatarsal. Note the extensive tophaceous soft tissue mass involving the medial, more than the lateral, aspect of the joint. B,C) Larger tophus in a different patient, with relatively little erosive change in the first MTP joint.
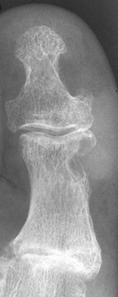
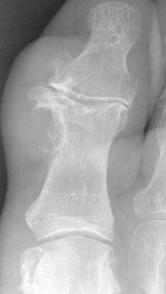
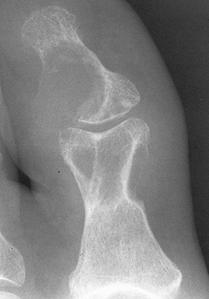
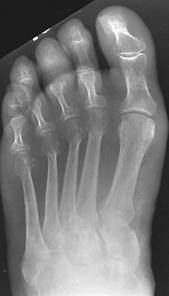
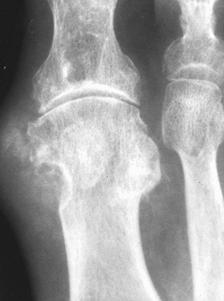
FIGURE 5-55 Gouty tophi. A–C) Tophi are associated with varying degrees of characteristic bone erosion. The tophi can eventually erode the underlying bone, producing punched-out lytic lesion with overhanging edges of bone and well-defined, sharply marginated sclerotic borders.D) Erosions due to tophi can result in significant bony destruction, even the disappearance of phalanges, as in this case involving the third toe.E) Tophi may calcify, distinguishing it from a rheumatoid nodule.
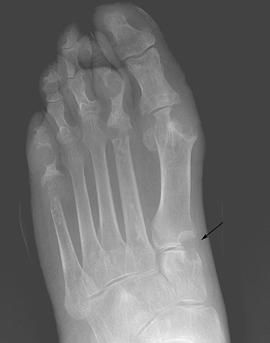
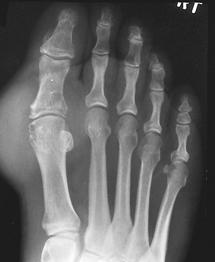
FIGURE 5-56AP radiograph demonstrating erosions with overhanging edges at the first TMT joint. There are also extensive erosions in the first MTP joint with near complete destruction of the second phalanges, second and fifth metatarsal heads, and the fifth proximal phalanx.
Involved digits exhibit eccentric and asymmetric joint swelling. Joint space loss is often absent, but there can be asymmetric joint space loss late in the disease process [16,69]. When the joint space is narrowed, the changes may resemble rheumatoid arthritis or erosive osteoarthriltis [16]. Bone mineralization is generally normal, but occasionally there may be mild osteopenia [14]. Although bony proliferation resulting in overhanging edges is typical of gout, osteophytes are not characteristic of gout. Chronic gout often results in an enlarged, irregular first metatarsal head. Malalignment and subluxations are rare in gout, but hallux valgus deformity is observed frequently.
Gout in the mid or hindfoot and ankle can present a confusing picture if there are no changes in the forefoot (Fig. 5-57). The ankle joint is rarely afflicted with gout, but posterior calcaneal erosions are common (Fig. 5-58). Retrocalcaneal bursitis is associated with soft tissue swelling, Achilles tendon thickening, and erosions of the posterior tendon secondary to tophi. In addition, the Achilles tendon can undergo fusiform or nodular enlargement and may eventually rupture [67]. TMT joint erosions may be marked. The monosodium urate crystals can inflict an intense inflammatory reaction, resulting in enhancement of tendon sheaths, ligaments, muscles, and bone marrow [14,16,56,84].
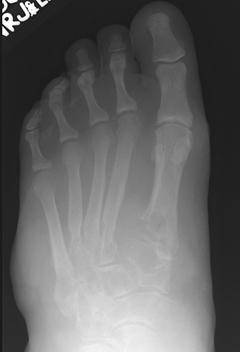
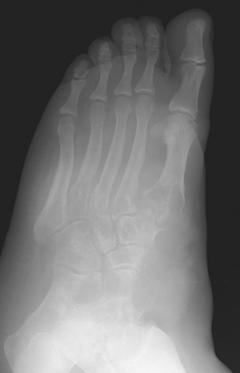
FIGURE 5-57 AP (A) and oblique (B) radiographs demonstrating extensive bone destruction in the midfoot with lobular soft tissue swelling. The forefoot is spared. This picture could be worrisome for pigmented villonodular synovitis or neoplasm.
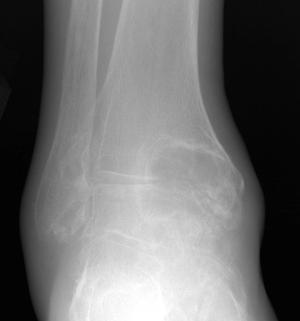
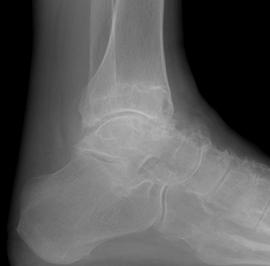
FIGURE 5-58 Gout in the ankle. AP (A) and lateral (B) radiographs of the ankle demonstrating large erosions in the medial maleollus and talus. There is a smaller intraosseous lesion in the distal fibula.
Though radiographs are most often used to follow patients with chronic gout, ultrasound, and CT and MR imaging are also more commonly used today [6,10,18,25,46,56,75,95]. Ultrasound is readily available, is less expensive, and in experienced hands can provide useful information regarding joint and synovial changes as well as demonstrate tophi in the soft tissues. Gouty tophi are typically nodular with heterogeneous hyperechoic appearance on ultrasound images [18,25]. In addition, ultrasound is very useful for joint aspiration to identify the typical crystals associated with gout [75].
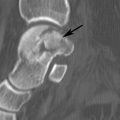
In our practice, CT is not commonly used to evaluate and follow patients with gout. However, osseous erosions and bone and soft tissue tophi can be easily identified on CT images (Fig. 5-59). Interosseous lesions containing calcification are clearly demonstrated on CT images. Gerster et al. [28] demonstrated that tophi with Hounsfield units greater than or equal to 160 may be diagnostic for gout.
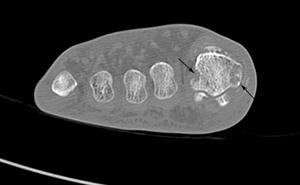
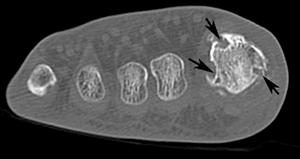
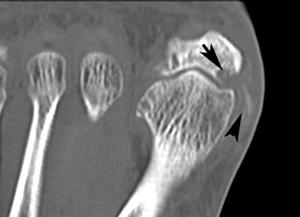
FIGURE 5-59 CT demonstrating gout in the first MTP joint. Axial images (A,B) demonstrate multiple erosions (arrows).C) Coronal image demonstrates an erosion (arrow) and calcifications in the medial soft tissues.
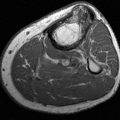
MR imaging reflects the changes caused by the proliferative synovitis (Fig. 5-60). Tophi are almost universally isointense to muscle on T1-weighted imaging, but they can have variable T2-weighted characteristics depending on the degree of calcification (Fig. 5-61) [6,10,56,95]. The T2-weighted signal is most commonly heterogeneously low to intermediate, but it can range from homogeneously increased to near-homogeneously decreased [95]. Contrast enhancement is variable, but peripheral enhancement is more commonly identified (Fig. 5-61) [10]. Although it is unusual to have tophi without prior episodes of gouty arthritis, soft tissue inflammation or masses without articular disease can be a diagnostic dilemma; tophi could be mistaken for neoplasm. Tophaceous gout should be considered when there is a heterogeneous low-to-intermediate signal mass associated with adjacent osseous erosive changes in the right clinical setting [6,10,56,95].
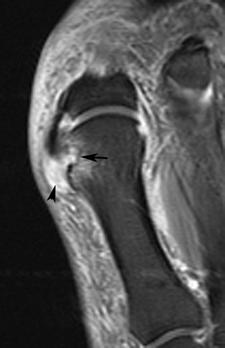
FIGURE 5-60 MR image in early gout. Coronal fast suppressed fast spin-echo T2-weighted image demonstrates and erosion with marrow edema (arrow) and a high signal inhomogeneous tophus medially (arrowhead).
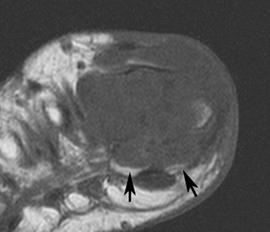
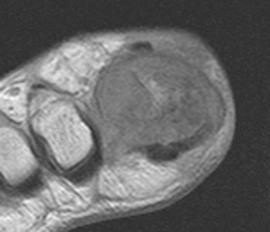
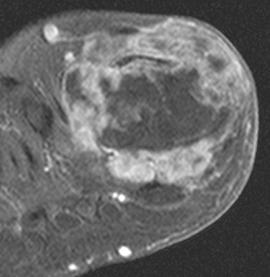
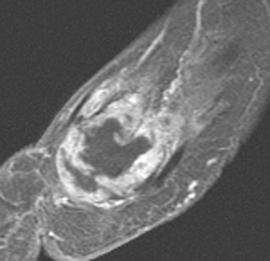
FIGURE 5-61 More advanced changes of gout involving the first MTP joint. Axial T1-weighted (A) and proton density-weighted (B) images demonstrate signal intensity isointense to muscle on the T1-weighted sequence and intermediate density on the proton density image. Note the involvement of both sesamoids (arrows). Postcontrast fat-suppressed T1-weighted images in the axial (C) and sagittal (D) planes demonstrate inhomogeneous and predominately peripheral enhancement.
Changes are often nonspecific with MR imaging [6]. Therefore, comparison with radiographs is essential to clarify the disease process (Fig. 5-62).
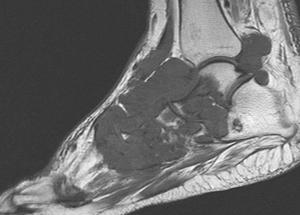
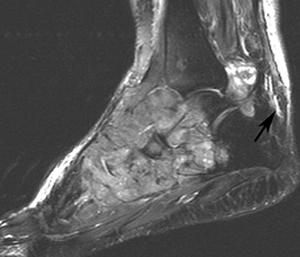
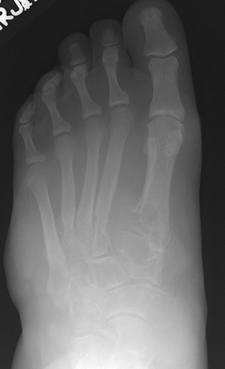
FIGURE 5-62 Aggressive changes of gout in the midfoot and ankle. Sagittal T1- (A) and T2-weighted (B) images demonstrate aggressive bone destruction and soft tissue masses. C) AP radiograph of the foot shows advanced bone destruction with dense soft tissue masses.
Management of patients with gout is most often conservative with anti-inflammatory medications including steroids for acute joint inflammation. Systemic or intra-articular steroids are also effective. Injection and joint aspiration can be accomplished with fluoroscopic or ultrasound guidance [75]. Maintenance therapy with colchicine was once the standard, but it is now reserved for patients that cannot tolerate anti-inflammatory medications. Recent reports demonstrate that etoricoxib and indomethacin are equally effective for management of gouty arthropathy [23].
Calcium Pyrophosphate Dihydrate Deposition Disease (CPPD)
Chondrocalcinosis is due to deposition intra-articular deposition of calcium compounds. The most common form is CPPD, though calcium apatite, dicalcium phosphate dehydrate, and calcium oxalate may also cause choncrocalcinosis [16]. Deposition of calcium pyrophosphate dehydrate crystals in the joint can illicit an inflammatory response resulting in synovitis and arthropathy [9,10].
Clinically, the condition increases with age with a 5% incidence in patients over 70 years of age and increasing to 50% by 90 years of age [16]. CPPD is associated with other disorders including hyperparathyroidism (15%), hemochromatosis (41%), and hereditary spherocytosis [16].
A large percentage of patients with radiographic evidence of chondrocalcinosis or CPPD are asymptomatic. Calcium pyrophosphate dehydrate crystals are commonly present in joints in patients without symptoms, but subclinical inflammation is likely due to elevated cell counts and polymorphonuclear cells in the synovial fluid [77]. When symptoms occur, the CPPD can mimic gout, rheumatoid arthritis, osteoarthritis, neuropathic, and other arthropathies. Over the years, largely through the work of McCarty and colleagues, CPPD has been classified into five clinical patterns [16,65,74,77].
- Asymptomatic (lanthanic)
- Pseudo-osteoarthritis
- Pseudogout syndrome
- Pseudorheumatoid arthritis
- Pseudoneuropathic arthritis
The most common form is lanthanic or the asymptomatic group. These patients have radiographic evidence of chondrocalcinosis, but the involved joint is asymptomatic [16,65,74]. The pseudo-osteoarthritis group has clinical symptoms and radiographic features similar to osteoarthritis, with chondrocalcinosis present in the symptomatic joint. Symptoms may be chronic, with intermittent acute episodes. This form typically involves the knee, hand, and wrist [16,74].
The pseudo-osteoarthritis form demonstrates radiographic features similar to osteoarthritis with superimposed choncrocalcinosis (Fig. 5-63) [16,65,74]. This pattern is one of the most common accounting for 50% of patients with CPPD. The knee, hand, and wrist are most commonly affected in patients with pseudo-osteoarthritis [16,65].
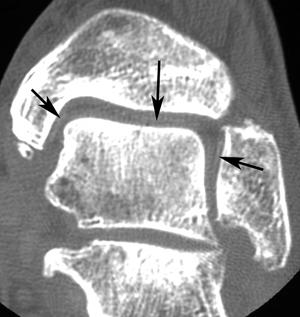
FIGURE 5-63 Coronal CT image of the ankle with changes of osteoarthritis and chondrocalcinosis (arrows).
The pseudogout form is a disorder that results from the excessive buildup or impaired degradation of pyrophosphate, leading to crystal deposition, synovitis, and arthritis. It usually manifests in the sixth and seventh decades by chondrocalcinosis and associated secondary osteoarthritis [71,74]. The frequency of calcium pyrophosphate dihydrate deposition is 1 in 1000 and increases with age [71]. This disorder can occur sporadically or in association with such metabolic disorders as hemochromatosis, phosphate and magnesium imbalances, hyperparathyroidism, and hypothyroidism. The weakly birefringent, variably shaped crystals can be recovered from synovial fluid, even in uninflamed joints, and intracellularly [77]. Calcification (Fig. 5-64) of cartilage, ligaments, and tendons is also common, particularly in the Achilles tendon. Pseuodogout can present with sclerosis, osteophytosis, cartilage loss, and subchondral cysts, similar to osteoarthritis. However, it differs from osteoarthritis in that it involves unusual joints, involves unusual sites within the affected joint, and exhibits prominent subchondral cysts, bone destruction, and variable osteophyte formation [33]. The arthropathy most commonly affects the tarsal and MTP joints, and the MTP joints may demonstrate capsular calcification.
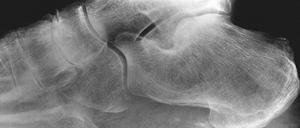
FIGURE 5-64 Calcium pyrophosphate dihydrate deposition (CPPD). Lateral image of the foot demonstrates calcification of the plantar aponeurosis and arthropathy involving the naviculocuneiform articulation.
The pseudorheumatoid arthritis form presents with symptoms similar to rheumatoid arthritis with morning stiffness and symmetrical distribution. The pseudoneuropathic group resembles changes of neuropathic arthropathy. In both instances the presence of chondrocalcinosis is a differentiating feature. Also, in the latter, there is no neurologic deficit [16,62,65,77].
Chondrocalcinosis in the foot and ankle may be subtle and difficult to detect on radiographs. CT is quite sensitive for detection of the subtle calcification (Fig. 5-63).
Hydroxyapatite Deposition Disease (HADD)
Calcium hydroxyapatite deposition commonly occurs in the tendons and bursae, but may also involve bone. The most common locations include the shoulder (69% of cases), followed by the hip, elbow, wrist, and knee in order of decreasing frequency (Fig. 5-65) [15,26,32]. In the foot, the region of the great toe and the flexor hallucis longus and brevis tendons are most commonly involved [32,97]. The hydroxyapatite may be seen as clumps, purple in color using Wright stain [16].
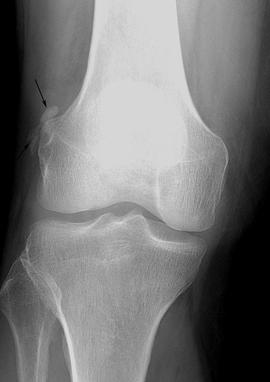
FIGURE 5-65HADD in a bursa about the knee. The calcification has a fluffy appearance (arrow) and there are no associated osseous changes.
Hydroxyapatite deposition disease is most common in patients 20 to 70 years of age [16,32]. Patients with calcium hydroxyapatite deposition may be asymptomatic and are more often sedentary workers. In one large series of over 6000 sendentary workers calcifications were noted in 2.7% [32]. The clinical phases have been divided into three phases. The silent phase when calcifications are contained within the tendon, the mechanical phase where the calcifications enlarge and may extend beyond the tendon and the adhesive periarthritis phase with pain and limitation of motion [32]. These phases are most commonly seen in the shoulder.
Images’ features are characteristic on radiographs in most cases (Fig. 5-65). Initially the calcifications may appear smoggy or cloud-like. In the later phases, the calcifications may appear more dense and organized. The shoulder is most commonly involved. Bilateral shoulder involvement occurs in up to 50% of cases [16,32]. Bone erosions are not commonly seen on radiographs. Intra-articular involvement has also been described, and it is not unusual to have calcium pyrophosphate crystals mixed with hydroxyapatite crystals [32,74].
MR images may demonstrate areas of low signal intensity corresponding to the calcifications demonstrated on radiographs. Comparison with radiographs is essential for accurate interpretation of the signal changes noted on MR. Areas of low signal intensity may be confused with tendon thickening on both T1- and T2-weighted sequences. Bone erosions and marrow edema may also be evident. Flemming et al. [27], noted cortical erosions in 76% and marrow edema in 36% of patients with HADD. When marrow edema is present without erosions the image features may be confused with infection, trauma, or neoplasm [15]. Other considerations include renal failure, tumoral calcinosis, gout, and CPPD [60].
Management can be difficult as the etiology is not completely understood [55]. In our practice, it is not unusual to aspirate the calcium, especially if it has as cloud-like radiographic appearance, and inject anesthetic and steroids. This can be accomplished with ultrasound or fluoroscopic guidance [75].
Hemochromatosis
Hemochromatosis is a chronic disease in which iron is excessively deposited in various tissues, resulting in parenchymal fibrosis, damage, and dysfunction. Hemochromatosis may be primary, which is a rare inherited disorder or secondary. Secondary hemochromatosis may follow cirrhosis, multiple transfusions, or excessive iron intake. In recent years, juvenile and neonatal forms have also been described [14,69]. Arthropathy occurs in 24% to 50% of patients with this disorder [14]. This disorder is more common in men and typically presents after 40 years of age [69].
Approximately half of the patients with hemochromatosis have arthritis similar to degenerative arthritis or CPPD [38]. The arthritis consists of osteopenia, subchondral cysts, bony eburnation, prominent chondrocalcinosis, beak-like osteophtytes, and loss of joint space [14,69]. Cystic lesions are subchondral and can become quite large. Sclerosis is due to subchondral trabecular thickening beneath eroded cartilage surfaces. The joint space narrowing is typically uniform [14]. Foot and ankle involvement occurs occasionally and does not differ in appearance from involvement elsewhere in the body (Fig. 5-66).
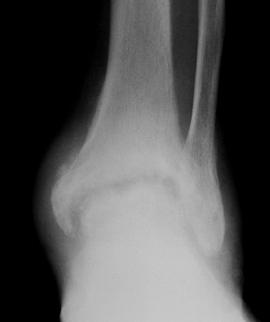
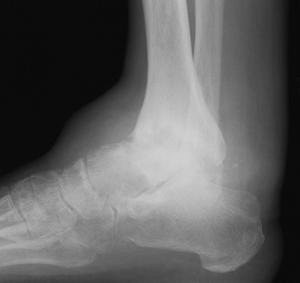
FIGURE 5-66 Hemochromatosis of the ankle. AP (A) and lateral (B) radiographs demon – strate joint space narrowing with coalescing subchondral cysts and bone sclerosis. There is a large ankle effusion.
COLLAGEN VASCULAR DISEASE
There are multiple connective tissue disorders including scleroderma, systemic lupus erythematosus, dermatomyositis, and mixed connective tissue diseases [14,69]. The upper extremities are more commonly involved, particularly the hands and wrists. This section focuses on systemic lupus erythematosus.
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a deforming, nonerosive (Jaccoud-like) arthropathy that can involve the foot and ankle. Articular involvement occurs in 75% to 90% of patients [14]. The articular deformities of lupus arthropathy are due to capsular and ligamentous laxity, contracture, and muscle imbalance as a result of intra-articular and periarticular inflammation and eventual fibrosis [69]. The arthropathy often is evident early in the disease, although it is more common and severe in patients with chronic disease. Typical findings include soft tissue swelling, regional osteoporosis, hallux valgus deformity (Fig. 5-67), subluxation of the MTP joints (Fig. 5-67), and widening of the forefoot [69]. Joint space narrowing, rare hook erosions of the metatarsal heads, subchondral cysts of the metatarsal heads, and osteonecrosis of the small bones of the foot can also be observed. Systemically or locally injected steroids can lead to tendon weakening and rupture, most commonly involving the Achilles tendon. Soft tissue calcifications can be diffuse, linear, streaky, or nodular in the subcutaneous and deeper soft tissues, plaque-like periarticular, or arterial. Patients with SLE are more prone to avascular necrosis, osteomyelitis, and septic arthritis of the ankle resulting from steroid use [69].
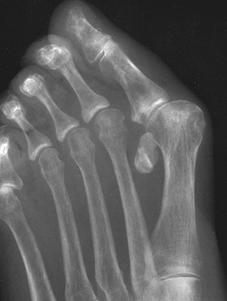
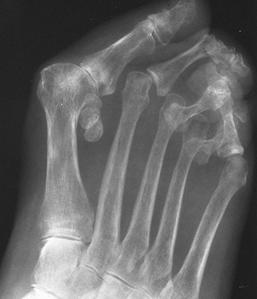
FIGURE 5-67 Systemic lupus erythematosus (SLE) of the foot. AP views of the (A) right and (B) left feet demonstrate bilateral hallux valgus deformities, fibular deviation, and subluxations of the left second through fifth MTP joints, and osteopenia. There are no erosions.
REFERENCES
- Ahmadi ME, Morrison WB, Carrino JA, et al. Neuropathic arthropathy of the foot with and without superimposed osteomyelitis: MR imaging characteristics. Radiology. 2006; 238:662–631.
- Alamanos Y, Voulgari PV, Drosis AA. Incidence and prevalence of psoriatic arthritis: a systematic review. J Rheumatol. 2008;35:1354–1358.
- Ansell BM. Juvenile rheumatoid arthritis, juvenile chronic arthritis, and juvenile spondyloarthropathies. Curr Opin Rheumatol. 1992;4:706–712.
- Ansell BM. Juvenile idiopathic arthritis. Current Orthop. 1998; 12:174–180.
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324.
- Ashman CJ, Klecker RJ, Yu JS. Forefoot pain involving the metatarsal region: differential diagnosis with MR imaging. Radiographics. 2001;21:1425–1440.
- Avila R, Pugh DG, Slocumb CH, et al. Psoriatic arthritis. A roentgenologic study. Radiology. 1960;75:691–701.
- Azouz EM, Duffy CM. Juvenile spondyloarthropathies. Clinical manifestations and medical imaging. Skeletal Radiol. 1995;24:399–408.
- Azouz EM. Juvenile idiopathic arthritis: how can the radiologist help the clinician? Pediatr Radiol. 2008;38(suppl 3): S403–S408.
- Bancroft LW, Peterson JJ, Kransdorf MJ. Imaging of soft tissue lesions of the foot and ankle. Radiol Clin North Am. 2008;46:1093–1103.
- Bezza A, Niamane R, Amine A, et al. Involvement of the foot in patients with psoriatic arthritis. A review of 26 cases. Joint Bone Spine. 2004;71:546–549.
- Bountry N, Morel M, Flipo R-M, et al. Early rheumatoid arthritis: a review of MRI and sonographic findings. Am J Roentgenol. 2007;189:1502–1509.
- Bouysset M, Tavernier T, Tebib J, et al. CT and MRI evaluation of tenosynovitis of the rheumatoid hindfoot. Clin Rheumatol. 1995;14:303–307.
- Brower AC, Flemming DJ. Arthritis in Black and White. 2nd ed. Philadelphia, PA: Saunders an Imprint of Elsevier; 1997.
- Bui-Mansfield LT, Moak M. Magnetic resonance appearance of bone marrow edema associated with hydroxyapatite deposition disease without cortical erosion. J Comput Assist Tomogr. 2005;29:103–107.
- Choi MH, MacKenzie JD, Dalinka MK. Imaging features of crystal-induced arthropathy. Rheum Clin North Am. 2006;32: 427–446.
- Chu C-H, Chen W-S, Wang T-G. Ultrasonographic presentations of tophi-like masses in atypical locations. J Med Ultrasound. 2006;144:35–39.
- Clouse ME, Gramm HF, Legg M, et al. Diabetic osteoarthropathy: clinical and roentgenographic observations in 90 cases. Am J Roentgenol. 1974;121:22–34.
- Cofield RH, Morrison MJ, Beabout JW. Diabetic neuropathy in the foot. Patient characteristics and patterns of radiographic change. Foot Ankle. 1983;4:15–22.
- Demetriades L, Strauss E, Gallina J. Osteoarthritis of the ankle. Clin Orthop. 1998;349:28–42.
- El-Khoury GY, Kathol MH, Brandser EA. Seronegative spondyloarthropathies. Radiol Clin North Am. 1996;34:343–357.
- Erdem CZ, Sarikaya S, Erdem LO, et al. MR imaging features of foot involvement in ankylosing spondylitis. Eur J Radiol. 2005;53:110–119.
- Fam AG. Treating acute gouty arthritis with selective COX 2 inhibitors. BMJ 2002;325:980–981.
- Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–336.
- Fessell DP, Jamadar DA, Jacobson JA, et al. Sonography of dordal ankle and foot abnormalities. Am J Roentgenol. 2003;181:1573–1581.
- Flemming DJ. Approach to the foot. In: Brower A, Flemming DJ, eds. Arthritis in Black and White. 2nd ed. Philadelphia, PA: W.B. Saunders; 1997:69–104.
- Flemming DJ, Murphey MD, Shekitka KM, et al. Osseous involvement in calcific tendinitis: a retrospective review of 50 cases. Am J Roentgenol. 2003;181:965–972.
- Gerster JC, Landry M, Dufresne L, et al. Imaging of tophaceous gout: computed tomography provides specific images compared to magnetic resonance imaging and ultrasonography. Ann Rheum Dis. 2002;61:52–54.
- Gladman DD, Mease PJ, Healy P, et al. Outcome measures of psoriatic arthritis. J Rheumatol. 2007;34:1159–1166.
- Gladman DD. Axial disease in psoriatic arthritis. Curr Rheumatol Rep. 2007;9:455–460.
- Hasler P. Biolgical therapies directed against cells in autoimmune disease. Semin Immunopathol. 2006;27:443–456.
- Hayes CW, Conway WF. Calcium hydroxyapatite deposition disease. Radiographics 1990;10:1031–1048.
- Hendry G, Gardner-Medwin J, Watt GF, et al. A survey of foot problems in juvenile idiopathic arthritis. Musculoskeletal Care 2008;6:221–232.
- Hodgson R, Grainger A, O’Connor P, et al. Dynamic contrast enhanced MRI of bone marrow edema in rheumatoid arthritis. Ann Rheum Dis. 2008;67:270–272.
- Hodgson RJ, Barnes T, Connolly S, et al. Changes in underlying dynamic contrast-enhanced MRI respose to treatment in rheumatoid arthritis. Skeletal Radiol. 2008;37:201–207.
- Holmes GB, Hill N. Fractures and dislocations of the foot and ankle in diabetics associated with Charcot joint changes. Foot Ankle Int. 1994;15:182–185.
- Jaakkola JI, Mann RA. A review of rheumatoid arthritis affecting the foot and ankle. Foot Ankle Int. 2004;25:866-874.
- Jensen PS. Hemochromatosis. A disease often silent but not invisible. Am J Roentgenol. 1976;126:343–351.
- Kamishima T, Tanimura K, Henmi M, et al. Power Doppler ultrasound of rheumatoid synovitis: quantification of vascular signal and analysis of interobserver variability. Skeletal Radiol. 2009;38:467–472.
- Karachalios T, Zibis A, Papanagiotou P, et al. MR imaging findings in early osteoarthritis of the knee. Eur J Radiol. 2004;50:225–230.
- Karasick D, Wapner KL. Hallux rigidus deformity: radiologic assessment. Am J Roentgenol. 1991;157:1029–1033.
- Kettering JM, Towers JD, Rubin DA. The seronegative spondyloarthropathies. Semin Roentgenol. 1996;3:220–228.
- Kippel JH, Stone JH, Crofford LJ, et al. Primer of Rheumatic Diseases. 13th ed. New York: Springer; 2007.
- Koh J, Dietz J. Osteoarthritis of the other joints (hip, elbow, foot, ankle, toes, wrist) after sports injuries. Clin Sports Med. 2005;24:57–70.
- Kucukdeveci AA, Kutlay S, Seckin B, et al. Tarsal tunnel syndrome in ankylosing spondylitis. Br J Rheumatol. 1995; 34(5):488–489.
- Kutilek S, bayer M, Dolezalova P, et al. Quantitative ultrasonometry of the calcaneus in children with juvenile idiopathic arthritis. Rheumatology. 2006;45:1273–1275.
- Liu S-Z, Yeh L-R, Chou Y-J, et al. Isolated intraosseous gout in the hallux sesamoid mimicking a bone tumor in a teen age patient. Skeletal Radiol. 2003;32:647–650.
- Lu DW, Katz KA. Declining use of the eponym “Reiter’s syndrome” in the medical literature, 1998–2003. J Am Acad Dermatol. 2005;53(4):720–723.
- Maksymowych WP. Update on the treatment of ankylosing spondylitis. Ther Clin Risk Manag. 2007;3:1125–1133.
- Marcus CD, Ladam-Marcus VJ, Leone J, et al. MR imaging of osteomyelitis and neuropathic osteoarthropathy in the feet of diabetics. Radiographics. 1996;16:1337–1348.
- Martel W, Stuck KJ, Dworin AM, et al. Erosive osteoarthritis and psoriatic arthritis. A radiologic comparison in the hand, wrist, and foot. Am J Roentgenol. 1980;134:125–135.
- Michelson J, Easley M, Wigley FM, et al. Foot and ankle problems in rheumatoid arthritis. Foot Ankle Int. 1994;15:608–613.
- Miller E, Uleryk E, Doria AS. Evidence-based outcomes of studies addressing diagnostic accuracy of MRI in juvenile idiopathic arthritis. Am J Roentgenol. 2009;192:1209–1218.
- Milner S. Common disorders of the foot and ankle. Surgery. 2006;24:382–385.
- Molloy ES, McCarthy GM. Hydroxyapatite deposition disease of the joint. Curr Rheumatol Rep. 2003;5:215–221.
- Monu JUV, Pope TL. Gout: a clinical and radiologic review. Radiol Clin North Am. 2004;42:169–184.
- Myerson MS, Henderson MR, Saxby T, et al. Management of midfoot diabetic neuroarthropathy. Foot Ankle Int. 1994;15: 233–241.
- Nishimura K, Sugiyama D, Yogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797–808.
- O’Dell J. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591–2602.
- Olsen KM, Chew FS. Tumoral calcinosis: pearls, polemics and alternative possibilities. RadioGraphics. 2006;26:871–885.
- Papa J, Myerson M, Girard P. Salvage, with arthrodesis, in intractable diabetic neuropathic arthropathy of the foot and ankle. J Bone Joint Surg. 1993;75A:1056–1066.
- Pascual E. The diagnosis of gout and CPPD crystal arthropathy. Br J Rheumatol. 1996;35(4):306–308.
- Peterson CC, Silbiger ML. Reiter’s syndrome and psoriatic arthritis. Their roentgen spectra and some interesting similarities. Am J Roentgenol. 1967;101:860–971.
- Petty RE, Southwood TR, Manner P, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision. J Rheumatol. 2004;31:390–392.
- Poor G. Crystal arthritis. Bailleres Clin Rheumatol. 1995;9: 397–406.
- Prieur AM. Chronic arthritis in children. Curr Opin Rheumatol. 1994;6:513–517.
- Reber P, Crevoisier Z, Noesberger B. Unusual localization of tophaceous gout. A report of four cases and review of the literature. Arch Orthop Trauma Surg. 1996;115:297–299.
- Reiter H. Ueber cine bisher unbekannte spirochaeten-infektion (spirochaetosis arthritica). Dtsch Med Wochenschr. 1916;42: 1535–1536.
- Resnick D, Kransdorf MJ. Bone and Joint Imaging. 3rd ed. Philadelphia, PA: Elsevier-Saunders; 2005.
- Reynolds PPM, Heron C, Pilcher J, et al. Prediction of erosion progression using ultrasound in established rheumatoid arthritis: a 2-year follow-up study. Skeletal Radiol. 2009;38:473–478.
- Rivera-Sanfeliz G, Resnick D, Haghighi P, et al. Tophaceous pseudogout. Skeletal Radiol. 1996;25:699–701.
- Roberton DM, Cabral DA, Malleson PN, et al. Juvenile psoriatic arthritis. Follow-up and evaluation of diagnostic criteria. J Rheumatol. 1996;23:166–170.
- Rosenberg ZS, Beltran J, Bencardino JT. MR imaging of the foot and ankle. Radiographics. 2000;20:S153–S179.
- Rosenthal AK, Ryan LM, McCarty DJ. Calcium pyrophosphate deposition disease, pseudogout and articular chondrocalcinosis. In: Koopman WJ, ed. Arthitis and Allied Conditions. 14 ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:2348.
- Saifuddin A, Abdus-Samee M, Mann C, et al. CT guided diagnostic foot injections. Clin Radiol. 2005;60:191–195.
- Salvarani C, macchioni P, Cremonesi T, et al. The cervical spine in patients with psoriatic arthritis: a clinical, radiological and immunognetic study. Ann Rheum Dis. 1992;51:73–77.
- Sanchis AM, Pascual E. Intracellular and extracellular CPPD crystals are a regular feature in synovial fluid from uninflammed joints of patients with CPPD related arthropathy. Ann Rheum Dis. 2005;64:1769–1772.
- Sanders TG, Linares R, Su A. Rheumatoid nodules of the foot. MRI appearances mimicking an indeterminate soft tissue mass. Skeletal Radiol. 1998;27:457–460.
- Schoellnast H, Deutschmann HS, Hermann J, et al. Psoriatic arthritis and rheumatoid arthritis: findings in contrast-enhanced MRI. Am J Roentgenol. 2006;187:351–357.
- Scranton PE Jr, McDermott JE. Anterior spurs: a comparison of open versus arthroscopic debridement. Foot Ankle. 1992;13:125–129.
- Sequeira W. The neuropathic joint. Clin Exp Rheumatol 1994; 12:325–337.
- Shereff MJ, Baumhauer JF. Hallux rigidus and osteoarthrosis of the first metatarsophalangeal joint. J Bone Joint Surg. 1998;80A:898–908.
- Sugimoto H, Takeda A, Masuyama J, et al. Early-stage rheumatoid arthritis. Diagnostic accuracy of MR imaging. Radiology. 1996;198:185–192.
- Surprenant MS, Levy AI, Hanft JR. Intraosseous gout of the foot. An unusual case report. J Foot Ankle Surg. 1996;35:237–243.
- Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis. Development of new criteria for a large international study. Arthritis Rheum. 2006;54: 2665–2673.
- Tehranzadeh J, Ashikyan O, Dascalos J, et al. MRI of large intraosseous lesions in patients with inflammatory arthritis. Am J Roentgenol. 2004;183:1453–1463.
- Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg. 2003;85A:923–936.
- Tong G, Sartoris DJ. Juvenile chronic arthritis: radiologic manifestations in the foot and ankle. J Foot Ankle Surg. 1996;35:260–262.
- Torshizy H, Hughes TH, Trudell D, et al. Anatomic features of metatarsal heads that may simulate erosive disease: cadaveric study using CT, radiography and dissection with special emphasis on sectional characterization of osseous anatomy. Am J Roentgenol. 2008;190:W175–W181.
- Trieb K. Management of the foot in rheumatoid arthritis. J Bone Joint Surg. 2005;87B:1171–1177.
- Tucker LB. Juvenile rheumatoid arthritis. Curr Opin Rheumatol. 1993;5:619–628.
- Van Rossum MAJ, Zwinderman AH, Boers M, et al. Radiologic features of juvenile idiopathic arthritis. A first step in the development of a standardized assessment method. Arthritis Rheum. 2003;48:507–515.
- Watt I. Osteoarthritis revisited-again! Skeletal Radiol. 2009;38:419–423.
- Weishaupt D, Schweitzer ME, Alam F, et al. MR imaging of inflammatory joint diseases of the foot and ankle. Skeletal Radiol. 1999;28:663–669.
- Yu JS, Chung C, Recht M, et al. MR imaging of tophaceous gout. Am J Roentgenol. 1997;168:523–527.
- Yu JS, Tanner J. Considerations in metatarsalgia and midfoot pain. Semin Musculoskelet Radiol. 2002;6:91–104.
- Zahrai A, Hossain D, Embil JM, et al. A man with an enlarging foot mass. CMAJ. 2004;171:1347.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree