6. Lung Ablation (Part II)
Advances in Thermal Techniques: Microwave Ablation
Since the first published report by Dupuy et al55 in 2000 on RFA of lung malignancies, new thermal energies have continued to evolve applications for tumor ablation within the chest. Of these, microwave energy1,2,56 appears promising, with many potential benefits emerging. Microwave ablation (MWA) is performed by induction of >900 MHz frequency, with the microwave energy inducing dipole excitation, which in turn causes the water molecules to spin, transferring some of their kinetic energy and creating friction, resulting in heat generation and tissue hyperthermia.1
When compared with RFA, the advantages of MWA include the ability to obtain higher intratumoral temperatures. The generator allows multiple applicators to be connected and simultaneously enacted, thereby decreasing ablation times and enabling larger ablation volumes. Microwave possesses an improved convection profile compared with radiofrequency, and less heat-sink effect has been seen with MWA when compared with RFA. No dispersive pads or grounding is necessary with microwave energy, lessening the chance of skin burns or short circuiting between pads and body parts. There is typically less pain on initiation of the procedure, likely from absence of current traveling through the intercostal nerves; less pain is associated with microwave for treatment of subpleural, juxtapleural, and chest wall lesions in close proximity to the somatically innervated parietal pleura and soft and osseous tissues of the chest wall.
Pearls: Microwave Ablation Advantages
Higher intratumoral temperatures
Larger ablation volumes
Less heat-sink effect
No grounding pads necessary
Less pain on initiation of the procedure and for peripheral and chest wall lesions
The disadvantages of MWA include higher cost of the microwave generator and antennae and unpredictable and larger ablation patterns, especially near surgical clips or staples at the target lesion. The antennae and the generator are more expensive. Implantable cardiac devices, including pacemakers and cardiac defibrillators, may be susceptible to interference from electromagnetic energy in the microwave frequency range, although less so than with radiofrequency energy. It is recommended to reprogram pacemakers to automatic pacing modes and temporarily disable implanted defibrillators during the procedure. External pacemakers can be used during temporary deactivation. Microwave antennae preferably should be positioned more than 5 cm from cardiac device leads, which can limit the area of ablation.
Pearls: Microwave Ablation Disadvantages
Higher cost of generator and antennae
Implantable cardiac devices may be susceptible to interference from electromagnetic energy
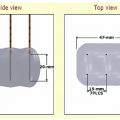
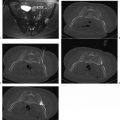
For MWA, the needle used is typically a 14.5-gauge microwave antenna, with an active tip of 1.6 or 3.7 cm and a length of 12, 17, or 22 cm; each antenna can be used alone or simultaneously with up to two additional antennae ( Fig. 6.24 ). Percutaneously placed under CT or CT-fluoroscopic guidance, the antennae are inserted into the tumor, and the ablation zone created is dependent on the duration of imparted energy and the number of antennae used. Specifically, the duration of imparted energy determines the size of the ablation zone in the plane perpendicular to and in the axis of the antenna and distal to the probe tip. For tumors larger than 2 cm in diameter, multiple antennae must be placed at up to 2-cm intervals within the tumor and no more than 1.5 cm from the lateral margins of the tumor, thereby ensuring not only proper ablational coverage of the tumor itself but an approximate 1-cm ablational margin around the tumor ( Fig. 6.25 ). Depending on the length of the applicator tip (1.6 or 3.7 cm), spherical zones of ablation up to 4.5 to 6 cm may be achieved in a single treatment ( Fig. 6.24 ). Actual treatment time typically varies from 7 to 10 minutes, with the longer treatment time conferring larger areas of thermal coagulation.
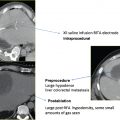
Once the microwave antenna(e) is in position, ablation is started and continued for 7 to 10 minutes via one, two, or three antennae and at each station if overlapping spheres are necessary for complete coverage of the tumor. In addition to manufacturer-recommended end times, the radiographic appearance and presence of ground glass encompassing the tumor with at least 5 mm but optimally greater than 10 mm of margin can be used to help determine the extent of coagulation necrosis ( Fig. 6.26 ). On follow-up imaging, the presence of cavitation within the treated lesion correlated with a reduced cancer-specific mortality.32 Typically, the MWA end point occurs at the end of a 10-minute cycle for single or multiple antenna(e) using 45 to 60 W of current. If needed, repeat overlapping ablations can be performed if these end points are not achieved. After completion of ablation, as with to radiofrequency, tract ablation of the lung parenchyma with 45 W may be performed. Attention must be paid to turning off the generator prior to the antenna tip reaching the extrathoracic soft tissues.
Similar to RFA, the most common immediate complication of percutaneous MWA within the lung is pneumothorax, with an incidence of 33 to 39%, approximately 33% of which requires chest tube placement.31,32 Intraprocedural skin burns with MWA occur in 3% of patients, with a majority of cases being mild; however, severe burns have been reported, requiring further intervention.31,32 Other complications include self-limited hemoptysis, empyema, and acute respiratory distress, all occurring less than 5% of the time.31,32 The post-ablation syndrome seen in RFA is also seen with MWA but to a lesser extent, with an incidence of approximately 2%.31,32 Again, treatment is typically symptomatic with antipyretics, pain control, and antitussives, on an as-needed basis. There have been no reports of intraprocedural or immediate post-procedural microwave-associated mortality.31,32 In one study, a single procedure-related mortality was documented that occurred approximately 9 months postablation secondary to overwhelming infection of the ablation cavity.31,32


Advances in Thermal Techniques: Cryoablation
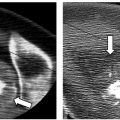
Cryoablation uses principles of cold-temperature dissipation to induce thermal injury in target tissues. The probes allow conduction of compressed argon gas in their interior hollow chambers that in turn leads to subzero temperatures in the active portion of the probe and surrounding tissues.2,56 Based on the duration of ablation and the diameter of the active probe, the size of the ice ball formed varies. The ice ball consists of various temperatures gradually increasing from the probe surface, which can reach temperatures as low as −150°C to the surface of ice ball at 0°C. Approximate isotherms are used and mapped by the vendors for probe selection. An innermost isotherm with temperatures of −40°C and an inner isotherm of −20°C are preferred zones for optimal ablation compared with an outer isotherm rim with temperatures ranging from −20° to 0°C ( Fig. 6.27 ). Cell death occurs at subzero temperatures by immediate post-thaw-freeze rupture, followed by long-term coagulation necrosis.
One of the primary advantages of cryoablation as compared with RFA is the freedom from imparting electric current, and thus it can be safely used in patients with pacemakers and implantable cardiac devices. Additionally, there is no disruption of the collagenous architecture, which has been found to be safer for nerves and bronchi with decreased associated pain. This is particularly beneficial when treating subpleural, juxtapleural, and chest wall tumors ( Fig. 6.28 ). Similar to radiofrequency and microwave devices, multiple probes can be used simultaneously, creating a larger zone of ablation in a shorter time period; the formed ice ball is easily visualized within soft tissues on CT, and thus the margins of the ablation zone are clearly demarcated against the margins of the tumor.



Pearls: Cryoablation Advantages
Safely used in patients with implantable cardiac devices
Decreased pain with subpleural, juxtapleural, and chest wall tumors
Larger ablation zones in a shorter time
Ablation zone control as the ice ball is easily visualized within the soft tissues
The disadvantages of cryoablation are poor visualization of the ice ball within the lung because of the low attenuation air surrounding the targeted tumor and parenchymal hemorrhage. Some authors have taken advantage of the pa-renchymal hemorrhage and perform an initial 2 minutes of cryoablation followed by thaw cycle to obtain peritumoral hemorrhage, for a better medium for larger ice ball formation. Pulmonary hemorrhage has been reported, not only occurring during the thaw cycle but especially on repositioning of the cryoprobe after the initial cryoablation cycle. Parenchymal hemorrhage within the lung can be disconcerting at the very least and troublesome enough that performance of cryoablation under general anesthesia has been advocated. Lastly, the gas containers are cumbersome and need to be checked for adequate pressure prior to every procedure.
Pearls: Cryoablation Disadvantages
Poor visualization of the ice ball within the lung
Equipment set-up, as it requires large tanks of argon and helium gas
Pulmonary hemorrhage during thaw cycle and on reposition of the cryoprobe after initiation of cryoablation
Current cryotherapy equipment consists of an argon and helium gas-based system with cryoprobes (CryoCare; Endocare, Irvine, CA; Fig. 6.29 ) that produce different-size ice balls depending on the size of the active tip ( Fig. 6.27 ). Cryoablation probe selection and number depends on the size and shape of the lesion. The innermost isotherm with temperatures of −40°C is lethal for fibrous tumors, whereas the inner isotherm with temperatures of −20°C is lethal for water-containing tumors. The cryoablation probes are placed in a manner to sculpt an ice ball that encompasses the tumor within the −20°C isotherm. Similar to both RFA and MWA, multiple overlapping probes 2 cm apart can be used for larger lesions. Utilizing the “1-2 rule” of probe placement, adequate ice coverage can be assured for most symmetric tumors where probes are placed 2 cm apart and 1 cm from the surface edge. A synergy is seen on the isotherms when multiple probes are used, where the cytotoxic isotherms produced by multiple probes have a larger area than a single probe, allowing for more reliable and thorough tumor coverage ( Fig. 6.6 ). The goal is to achieve homogeneous coverage of the tumor within the inner −20° isotherm as well as an adjacent 5-mm, but again ideally greater than 10-mm, margin of nontumorous lung tissue. Once the probes are in place, a typical cryoablation includes a 10-minute freeze phase, followed by a 5- to 8-minute stick/thaw phase, followed by a second 10-minute freeze phase. Once the probes are in place and a cryoablation cycle has begun, the probes should not be repositioned, for the risk of significant bleeding increases ( Fig. 6.30 ). However, a probe can be withdrawn along the course of its insertion, and therefore a second cycle of freeze/thaw phases can be performed for portions of the target tumor that were not included in the inner isotherm on the first cycle of cryoablation. This is useful in tumors that spread in a contiguous fashion as is seen in recurrent chest wall mesothelioma ( Fig. 6.31 ). Finally, the probes can be removed once the probe temperature reaches 20°C.


Pearls: Cryoablation Procedure
Probe selection and number depends on size and shape of the lesion.
Fibrous tumors must lie within the −40°C isotherm and water-containing tumors within the −20°C isotherm to achieve complete cryonecrosis.
Multiple probes:
“1-2” rule: probes should be placed 2 cm apart and 1 cm from the surface edge.
Synergy is seen with multiple probes, where the cytotoxic isotherms have a larger area than that produced from a single probe.
Cryoablation includes a 10-minute freeze phase, followed by 8-minute stick/thaw phase, followed by a second 10-minute freeze phase.
Probes can be removed once the probe temperature reaches 20°C.

The most common complications reported in a study by Wang et al2 were self-limited and did not require intervention; they included cough, hemoptysis, fever, and hypertension. Pleural effusions were seen in 14% of patients and seen more frequently with pleural-based lesions; however, only 5% required drainage. Pneumothoraces occurred in 12% of patients, with 46% requiring periprocedural evacuation and 12% requiring catheter drainage. Special attention should be paid to self-limited hemoptysis that occurred in 62% of patients, much higher when compared with radio-frequency and MWA. Although these cases were self-limited and typically ceased within 1 week of therapy, it is prudent to warn both patients and the support staff, so as to reduce intra- and postprocedural anxiety and to make the necessary preparations (spit basin and suction). Another complication that occurred at an increased frequency relative to RFA and MWA was the presence of self-limited mild to moderate hypertension. However, special attention should be paid to patients with preexisting hypertension who may require more aggressive monitoring and additional blood pressure control. Less common complications included subcutaneous emphysema and skin and neural injury (brachial plexus and recurrent laryngeal), all occurring less than 5% of the time. When using cryoablation in the chest wall, skin involvement may result in frost bite, and appropriate measures and precautions must be undertaken to protect the cutaneous tissues during freezing. Although no intraprocedural deaths occurred, two postprocedural deaths have been reported secondary to acute respiratory distress syndrome and as a result of a pulmonary embolus. The postablation syndrome seen in RFA, and to a lesser extent, in MWA has not been reported in cryoablation patients; however, mild to moderate fever is commonly seen and may be treated with antipyretics as needed.2
Pearls: Cryoablation Precautions
Bowel precautions
Air/saline displacement: Using a small-bore catheter, air and nonionic sterile water can be instilled into the peritoneum to provide adequate distance between the edge of the ice ball and adjacent bowel ( Fig. 6.32 ).
Skin precautions
A sterile glove filled with warm water is placed on the skin overlying the target lesion to prevent skin necrosis.
Infusion of a mixture of sterile water and lidocaine into the subcutaneous fat can increase the distance between the target lesion and skin.
Postprocedure
Following thermal ablation, patients need to be observed for potential complications from anesthesia and ablational effects. Patients should be observed and monitored in the post-anesthesia care unit with regular vital signs and continuous pulse oximetry. An upright expiratory chest radiograph should be obtained within 2 hours of the procedure, and a second expiratory chest radiograph should be obtained at 3 to 4 hours postprocedure. Chest radiographs are obtained to exclude immediate complications, such as a pneumothorax, pleural effusion, hemothorax, and pulmonary infiltrates. Hemoglobin and hematocrit levels can be obtained at 3 to 4 hours postprocedure to screen for occult hemorrhage, if desired. Postprocedure pain control can include either oral analgesics or patient-controlled analgesia pumps for parenteral administration of narcotics. Use of antiinflammatory agents, such as ibuprofen, is recommended to suppress postablation inflammation, particularly with RFA, and to a lesser extent MWA, and prescribed for at least 5 days postprocedure; these agents may decrease the degree of pain, pleural effusion, and systemic inflammatory response. Postprocedural low-grade fever for 2 to 3 days is common.

Depending on the patient’s clinical course and assessment, the operator determines whether limited or overnight admission for observation is required. Typically, patients can be discharged within 1 to 2 days after thermal ablation, unless a thoracostomy drain was required. Many operators, however, routinely discharge patients on the same day, with strict postprocedure instructions and close follow-up. Patients should generally be instructed not to travel by air for at least 3 weeks. Airplane cabins are not fully pressurized to sea level, and exposure to this environment could potentially exacerbate a known or an occult pneumothorax.
Pearls: Postprocedure
Postanesthesia care unit observation and monitoring for possible procedural or anesthesia complications
Chest radiographs: One within 2 hours and a second within 3 to 4 hours to evaluate for pneumothorax, pleural effusion, hemothorax, and pulmonary infiltrates
Hemoglobin and hematocrit levels at 3 to 4 hours post-procedure to screen for occult hemorrhage
Pain control with oral or intravenous medications
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree