PART VI Trauma
A 29-year-old man presents with progressive chemosis, proptosis, and ophthalmoplegia of the left eye 1 week after sustaining head trauma in a motorcycle accident. He also has pulsatile tinnitus that began the first day after the accident. His neurologic examination is normal. CT and angiography are performed.
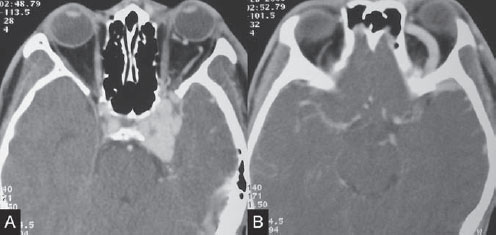
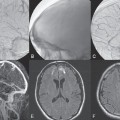
Fig. 42.1 Contrast-enhanced CT scan shows left-sided exophthalmos with bulging of the left cavernous sinus, (A) left IOV, and (B) left SOV. Prominence of the left spheno-parietal sinus along the anterior aspect of the middle cranial fossa is noted.
Contrast-enhanced CT study showed bulging of the left cavernous sinus with a dilated left superior ophthalmic vein (SOV) and inferior ophthalmic vein (IOV). Exophthalmos of the left orbit was noted with deviation of the left globe medially, likely related to the patient’ s ophthalmoplegia. There was prominence of the left sphenoparietal sinus along the anterior aspect of the middle cranial fossa, suggestive of cortical venous reflux (Fig. 42.1).
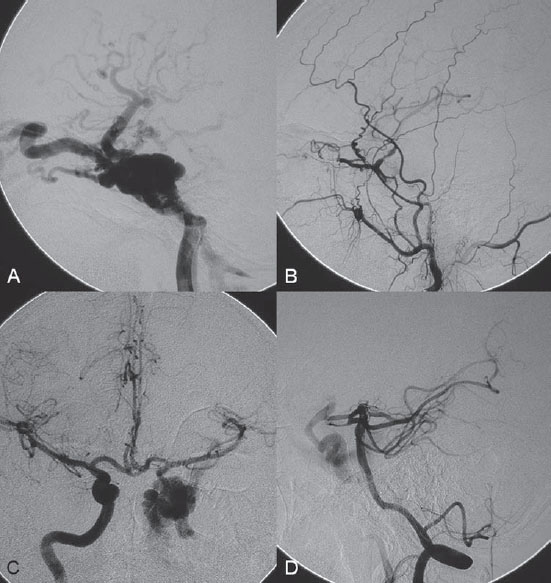
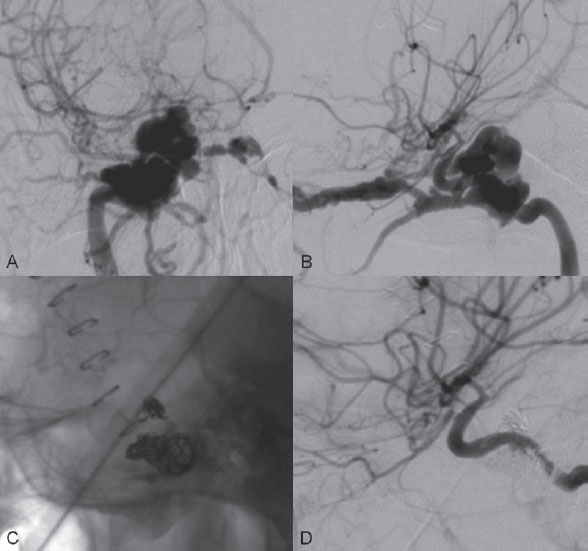
Injection of the left internal carotid artery (ICA) showed a direct high-flow arteriovenous fistula from the cavernous portion of the left ICA to the left cavernous sinus. There was subsequent drainage through the left SOV and left IOV, with cortical venous reflux via the left sphenoparietal sinus to the left superficial sylvian vein and the left deep sylvian vein to the left basal vein of Rosenthal. Collaterals from the left middle meningeal artery to the left ophthalmic artery (left meningo-ophthalmic artery) were seen on lateral view of the left external carotid artery (ECA) injection, with retrograde filling of the left ICA. Enlargement of the left artery of the foramen rotundum from the distal internal maxillary artery, serving as a collateral to the left ICA, was also observed. The right ICA injection with compression of the left common carotid artery (CCA) in AP view showed rather good collateral flow through the anterior communicating artery. There was moderate size of the left posterior communicating artery, seen on the left vertebral artery (VA) injection with compression of the left CCA in lateral view. The exact site of the fistula was best demonstrated on this projection (Fig. 42.2).
Fig. 42.2 DSA. (A) Left ICA angiogram in lateral view. (B) Left ECA angiogram in lateral view shows the left meningo-ophthalmic collateral and the artery of the foramen rotundum ICA collateral, with faint opacification of the left ICA. (C) Right ICA angiogram in AP view and (D) left VA angiogram in lateral view during left CCA compression. Note that the actual site of the fistula is best seen from the left VA injection.
Left traumatic carotid-cavernous fistula (TCCF) with cortical venous reflux
- Standard 8F access (puncture needle, 8F vascular sheath)
- Standard 8F guiding catheter (Cordis, Warren, NJ) with continuous flush and a 0.038-in hydrophilic guidewire (Terumo, Somerset, NJ)
- No. 9 gold valve detachable balloons (Nycomed, Melville, NY) and GOLDBAL5 gold valve detachable balloon (Balt International, Montmorency, France)
- Microcatheter system (Baltacci microcatheters for detachable balloons; Balt)
- Isotonic contrast solution for balloon inflation
- Contrast material
- Heparin 3000 units, protamine 30 mg
After diagnostic angiography, 3000 units of heparin were given intravenously. The 8F guiding catheter was advanced carefully into the proximal left ICA. (A coaxial system may be used to facilitate navigation of the large guiding catheter and to avoid possible dissection of the ICA.) The detachable balloon was prepared and mounted onto the microcatheter system. A roadmap was done, followed by introduction of the balloon and detachable system into the guiding catheter. After the balloon was within the left ICA, it was slightly inflated with 0.1 mL of isotonic contrast solution to facilitate navigation into the fistula. After the balloon entered the cavernous sinus, it was slightly inflated while several injections via the guiding catheter were performed to check its position. When the balloon was correctly placed within the first venous pouch, closest to the left ICA, it was fully inflated and detached. The microcatheter was then removed, and a final control run was performed. The heparin was reversed with 30 mg of protamine at the end of the procedure. Unfortunately, the balloon deflated 2 days after the embolization, with early reopening of the TCCF. The patient underwent a second embolization 1 month later in which two balloons were placed in a similar procedure. The first balloon was navigated more distally within the cavernous sinus and detached while not fully inflated to facilitate placement of the second balloon, which was then detached fully inflated within the first venous pouch. Final control angiograms showed complete closure of the fistula, which was confirmed on follow-up MRA 2 months after the second embolization session (Fig. 42.3). The patient was prescribed absolute bed rest for 3 days before being discharged home and was advised to limit his activities for at least 1 month to avoid dislocation of the balloons.
The most common cause of a direct carotid-cavernous fistula is trauma. A TCCF may also be iatrogenic, usually following skull base surgery, or result from rupture of an underlying cavernous aneurysm or a disease of the vascular wall, such as Ehlers-Danlos syndrome or neurofibromatosis type 1. The pathologic mechanism of a TCCF is tearing and rupture of the ICA, which is fixed at the cavernous level by fibrous trabeculae and by small dural arteries into the cavernous sinus. The clinical symptoms depend on the drainage pathways. Anterior drainage toward the SOV and IOV will lead to exophthalmos and chemosis; posterior drainage into the inferior petrosal vein results in pulsatile tinnitus; and cortical venous reflux through the sphenoparietal sinus into the superficial middle cerebral vein or the superior petrosal vein into the posterior fossa may cause venous congestion with subsequent delayed hemorrhage.
- A loud bruit at the orbit or region behind the ear together with a history of significant head trauma makes the diagnosis.
- Exopthalmos and chemosis are classically seen.
- Because a TCCF can be diagnosed from the clinical history and physical examination, CT and MRI are used mainly to rule out other possible causes of chemosis and proptosis and associated brain injuries.
- Bulging of the cavernous sinus and dilatation of the SOV are best seen on contrast-enhanced CT or T2-weighted images.
- The presence of an enhancing lesion within the sphenoid sinus in the context of a TCCF suggests an associated sphenoid pouch/pseudoaneurysm, a situation in which emergency treatment is indicated.
- Cortical venous reflux is typically seen as prominence of the sphenoparietal sinus located along the anterior aspect of the middle cranial fossa and dilated vessels along the sylvian fissure or cerebellar folia.
- CT, MRI, and static CTA and MRA cannot identify the side or exact site of the fistula. Static CTA and MRA may not be helpful because of the high-flow nature of the TCCF and multiple drainage routes.
- Dynamic CTA and dynamic MRA may be able to identify the side of the fistula, which is seen as early filling of the cavernous sinus.
- Angiography is required in the pre-treatment evaluation of TCCFs and may be performed in the same session as the endovascular treatment.
- Both CCAs should be checked first with hand injections before the catheter is advanced into the ICAs to look for associated arterial dissection.
- The ICA angiogram on the suspected side of the TCCF is performed first, in both AP and lateral views, to determine the drainage pathways of the fistula. The ipsilateral ECA is then injected to rule out associated vascular injuries and evaluate the ophthalmic artery collaterals in case the ICA must be sacrificed.
- The contralateral ICA angiogram is performed in AP view during manual compression of the ipsilateral CCA to evaluate the collateralization through the anterior communicating artery. This is followed by injection of the VA in lateral view, also during manual compression of the ipsilateral CCA, to evaluate the size of the posterior communicating artery. The exact site of the fistula is usually best seen on the lateral view of the VA angiogram.
- In the past, TCCFs were treated with carotid ligation or muscle embolization through surgical exposure of the carotid artery. Because this was often done blindly, the procedure was associated with a high risk for complications and low cure rates; therefore, it is rarely practiced nowadays.
- Other surgical options include direct packing of the carotid-cavernous fistula and surgical disconnection of the cortical venous reflux.
- Balloon embolization is the first choice in the treatment of TCCFs. If balloons are not available or the tear in the ICA is too small, then platinum coils (see Case 45) and/or liquid embolic materials may be considered.
- Heparin is routinely given to all patients; it is reversed with protamine at the end of the procedure.
- The goal of treatment is to close the first venous pouch, which is the one closest to the ICA tear.
- If more than one balloon is required, then the first balloon is navigated further into the cavernous sinus and detached not fully inflated.
- In our institution, all patients are prescribed absolute bed rest for at least 72 hours after the procedure to avoid migration of the balloon. Aggressive medication for nausea, vomiting, hiccups, and coughing may be needed.
- If early deflation of the balloon occurs within this period, then the bed rest is cancelled and another embolization is rescheduled after 1 month. This waiting period is often necessary to allow the first balloon to deflate enough that placement of the second or third balloon will not be obstructed.
- If the TCCF recurs after the second embolization, the period of bed rest may be extended to 5 days, or other embolic materials, such as coils and/or liquid agents, are considered.
- After discharge from the hospital, patients are usually advised to limit their activities for at least 1 month following the procedure.
- Standard angiographic complications (at the puncture site: bleeding, false aneurysms, fistula; in catheterized vessels: emboli, dissections; systemically: contrast reaction, renal failure)
- Migration and/or early deflation of the balloon
- Transient worsening of ophthalmoplegia and exophthalmos occurs after complete closure because of progressive thrombosis of the SOV, which usually regresses after medical management with steroids
Published Literature on Treatment Options
In 1981, Debrun et al published the first large series of patients with TCCFs treated with detachable balloon embolization via both transarterial and transvenous approaches. Since then, embolization has replaced the older surgical methods, with the advantages of a lower risk for complications, higher rate of ICA preservation, and much higher cure rates. In a large series of 100 patients reported by Lewis et al, the cure rate was 86%, and the ICA was preserved in 75% of cases, with a complication rate of only 4%. Many cases in which different techniques were used have been reported, but in our opinion, the easiest route is still a transarterial route with a detachable balloon. If this fails, other embolic materials, such as coils and liquid agents, or a transvenous route may be considered. Reports of successful reconstruction of the ICA with covered stents have recently been published by several groups, and this may be the ideal treatment in the future; currently, however, there are still no dedicated covered stents for intracranial use, and the long-term patency of these stents has yet to be determined.
PEARLS AND PITFALLS__________________________________________________
- TCCFs are diagnosed clinically by the history and the presence of a bruit. CT, MRI, and static CTA/MRA cannot determine the side and site of the fistula.
- A medial sphenoid pouch, like a sphenoid pseudoaneurysm, is an indication for emergency treatment, given the risk for life-threatening epistaxis.
Debrun G, Lacour P, Vinuela F, Fox A, Drake CG, Caron JP. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg 1981;55(5):678–692
Gupta AK, Purkayastha S, Krishnamoorthy T, et al. Endovascular treatment of direct carotid cavernous fistulae: a pictorial review. Neuroradiology 2006;48(11):831–839
Lewis AI, Tomsick TA, Tew JM Jr. Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery 1995;36(2): 239–244, discussion 244–245
Wang C, Xie X, You C, et al. Placement of covered stents for the treatment of direct carotid cavernous fistulas. AJNR Am J Neuroradiol 2009;30(7):1342–1346
Clinical Presentation
A 34-year-old man is admitted to the hospital with the history of a severe head injury and loss of consciousness following a motor vehicle accident. Initial CT scan shows crushing skull base fractures and traumatic sub-arachnoid hemorrhage. His initial neurologic examination reveals no motor or sensory deficits. He has massive facial swelling related to the extensive facial and skull base fractures, so that the clinical development of progressive right proptosis is not noticed. Two weeks after admission, the new onset of left hemiparesis is noted. Another CT scan is performed, followed by angiography.
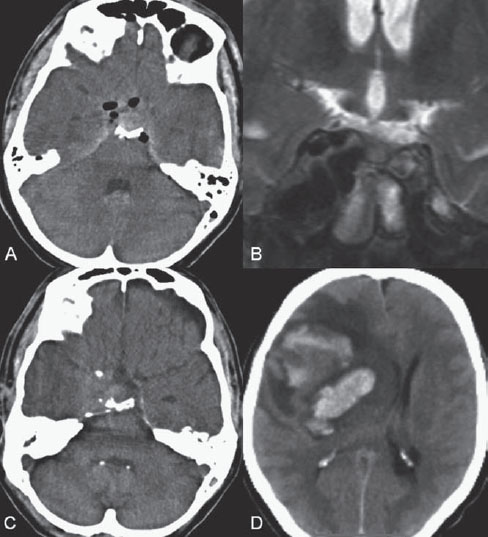
Fig. 43.1 (A) Initial unenhanced CT shows hyperdensity along the suprasellar cistern and the hypodensity of air within the subarachnoid space and right cavernous sinus. (B) Follow-up coronal T2-weighted MRI 1 week later demonstrates bulging of the right cavernous sinus with multiple internal flow-void structures. (C) Follow-up unenhanced CT at 2 weeks reveals bulging of the right cavernous sinus at the level of the skull base. (D) Unenhanced CT done one day later after the acute onset of left hemiparesis shows a large hyperdense hematoma involving the right fronto-temporal lobe and basal ganglia with marked surrounding hypodensity due to edema.
Initial unenhanced CT showed traumatic subarachnoid hemorrhage and air within the subarachnoid space and right cavernous sinus. Multiple fractures at the right side of the skull base were noted. Follow-up MRI 1 week later showed bulging of the right cavernous sinus with multiple internal flow-void structures on T2-weighted images. The follow-up unenhanced CT study at 2 weeks after admission, when the patient exhibited neurologic deterioration, revealed a new, large intraparenchymal hemorrhage along the right frontotemporal lobe and basal ganglia, with marked surrounding edema and mass effect. Bulging of the right cavernous sinus was noted at the level of the skull base. These findings suggested the diagnosis of a traumatic carotid-cavernous fistula (TCCF) with cortical venous reflux and secondary venous infarction (Fig. 43.1).
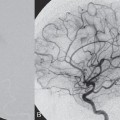
Injection of the right internal carotid artery (ICA) showed a direct high-flow arteriovenous fistula from the cavernous portion of the right ICA to the right cavernous sinus. Drainage through the right superior ophthalmic vein (SOV) and right inferior ophthalmic vein (IOV) with cortical venous reflux via the right sphenoparietal sinus to the right frontal cortical veins and the right deep sylvian vein toward the right basal vein of Rosenthal was noted (Fig. 43.2 A,B). Poor collateralization through the anterior communicating artery and right posterior communicating artery was observed (not shown).
Right TCCF with cortical venous reflux
- Standard 8F access (puncture needle, 8F vascular sheath)
- Standard 8F guiding catheter (Cordis, Warren, NJ) with continuous flush and a 0.035-in hydrophilic guidewire (Terumo, Somerset, NJ)
- GOLDBAL1 gold valve detachable balloon (Balt International, Montmorency, France)
- Magic BD PE 1.8F catheter for detachable balloon (Balt)
- Isotonic contrast solution for balloon inflation
- A 0.019-in over-the-wire microcatheter (Excelsior 10–18; Boston Scientific, Natick, MA) with a 0.014-in hydrophilic guidewire (Synchro 14; Boston Scientific)
- Bare and fibered detachable platinum coils (GDC 360-degree SR10 and GDC VortX; Boston Scientific)
- Contrast material
- Heparin 5000 units
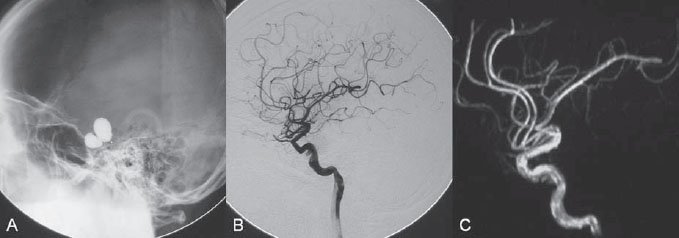
After diagnostic angiography, 5000 units of heparin was given intravenously. An 8F guiding catheter was advanced carefully into the proximal right ICA. A roadmap was done. Several attempts to place a small balloon into the fistula failed because of the small size of the tear within the right ICA. Two separate tears were identified with hand injections during gentle inflation of the balloon within the cavernous ICA. The balloon and microcatheter were removed, and an over-the-wire microcatheter was introduced into the first tear along the inferior wall of the right ICA. A 360-degree bare platinum coil was placed and detached in the first venous pouch as a frame, followed by careful placement of multiple fibered platinum coils to introduce further thrombosis until stagnation of the contrast within the cavernous sinus was observed on the hand injections. The microcatheter was then gently removed and maneuvered into the second tear within the superior wall of the right ICA. A frame was formed by placement of a 360-degree bare platinum coil within the first venous pouch closest to the ICA tear, followed by packing with fibered platinum coils until no filling of the cavernous sinus was seen on the hand injection. The microcatheter was then removed, and the final control run revealed complete obliteration of the fistula (Fig. 43.2 C,D).
Fig. 43.2 DSA. Right ICA angiogram in (A) AP and (B) lateral views shows a high-flow arteriovenous fistula from the cavernous portion of the right ICA to the cavernous sinus. (C) Plain skull radiography and (D) right ICA angiogram after coil embolization demonstrate the position of the two coil meshes and confirm complete closure of the fistula.
TCCFs occur secondary to rupture of the ICA or its cavernous branches. Occasionally, rupture of an intra-cavernous branch from the external carotid artery (ECA) may result in similar symptoms. Cavernous dural arteriovenous fistulae, also known as indirect carotid cavernous fistulae, are a separate entity (see Case 30). Significant head trauma is typically required for a TCCF; in some cases with no history of trauma or only minor trauma, an underlying lesion such as a cavernous aneurysm or a vessel wall disease should be suspected. The clinical symptoms are typically related to the drainage pathways. In rare instances, fracture of an adjacent wall of the sphenoid sinus may lead to the development of an associated sphenoid aneurysm and result in massive epistaxis; these situations are life-threatening emergencies. Aneurysmal dilatations of the venous pouches may also project intradurally, and subsequent rupture can produce subarachnoid hemorrhage. Intraparenchymal hemorrhage and venous infarctions are usually caused by cortical venous reflux through either the sphenoparietal sinus to the superficial sylvian vein or the superior petrosal sinus to the posterior fossa veins. Because the cavernous sinuses are anastomosed in the midline, TCCFs can produce symptoms contralaterally or bilaterally.
- In patients with crushing skull base fractures, proptosis or chemosis may be difficult to recognize. However, a bruit at the orbit or region behind the ear may help in making the diagnosis.
- A crushing skull base fracture that crosses the ICA canal as well as air within the ICA canal should raise suspicion of a carotid artery laceration.
- Progressive bulging of the cavernous sinus is a good indicator of the development of a TCCF.
- If a TCCF is suspected from the imaging findings, CTA or MRA is indicated to rule out cortical venous reflux.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree