7 Chronic Venous Occlusive Disease
Summary
Chronic venous occlusive disease (CVOD) is commonly treated conservatively by the medical community at large. Most patients with symptomatic CVOD are managed with long-term extremity compression garments, and often with chronic anticoagulation. Many patients suffer from lifestyle-limiting symptoms, and sequelae can include signs and symptoms of chronic venous stasis, with soft tissue changes or ulceration. This chapter familiarizes the reader with standards for evaluation and management of patients with symptomatic CVOD, including review of techniques for recanalization of occluded venous segments with tips for postprocedural longitudinal management of these patients.
7.1 Introduction
Chronic venous occlusive disease (CVOD) resulting from deep vein thrombosis (DVT) is characterized by a constellation of symptoms collectively known as postthrombotic syndrome (PTS). The characteristic chronic leg swelling, pain, skin changes, varicose veins, and venous stasis ulcers affect nearly 50% of patients with proximal iliofemoral DVT within the first 1 to 2 years of presentation. 1 CVOD results in significant morbidity as well as personal, emotional, and economic costs to the individual and society. 2 The vast majority of patients with CVOD never undergo treatment and most are never referred to a vascular specialist for management.
Most commonly, CVOD can broadly be categorized into those cases resulting from inferior vena cava (IVC) filter complication and those due to proximal DVT. The technical approach and medical management of these conditions are discussed herein.
7.2 Case Vignette 1
7.2.1 Patient Presentation
A 35-year-old woman developed acute left iliofemoral DVT 2 days postpartum, presenting with chronic left leg swelling, muscle fatigue, cramping, and intermittent left buttock pain. Warfarin therapy was prescribed for 6 months, followed by treatment with rivaroxaban (Janssen Pharmaceuticals, Rariton, NJ) for 3 months. Nine months following her initial presentation, due to ongoing symptoms of CVOD, a CT venogram was obtained and demonstrated significant left common iliac vein compression and thrombotic occlusion of the left external iliac vein. At that time, she underwent an unsuccessful attempt at recanalization of her left external iliac vein. It was recommended that she continue anticoagulation indefinitely and wear thigh-high compression stockings. Two years later, she presented for reevaluation. At that time, she reported significant swelling in the left lower extremity with failure to use compression. She was an avid runner and unable to run long distances or do prolonged physical activity due to pain and swelling.
7.2.2 Physical Exam
On physical examination, the patient was a slender woman with mild asymmetric enlargement of the left lower extremity, relative to the right. She had no obvious skin manifestations, varicosities, or ulcers.
7.2.3 Noninvasive Testing
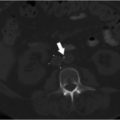
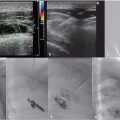
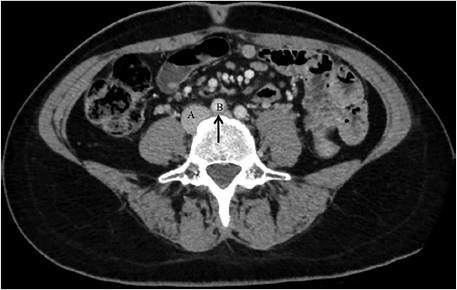
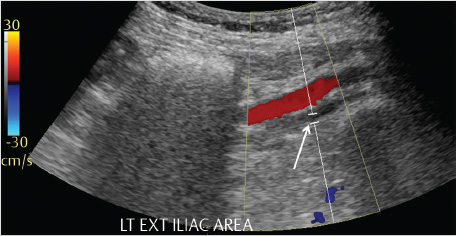
Review of the initial preoperative CT venogram from 2 years prior showed significant compression of the left common iliac vein by the overlying right common iliac artery, suggestive of May–Thurner syndrome (Fig. 7.1a; Fig. 7.1b). A duplex ultrasound (US) performed contemporaneous to the consult revealed occlusion of the left external iliac vein with patent common femoral, femoral, and popliteal veins (Fig. 7.2).
7.2.4 Invasive Testing
The catheter venogram from the prior recanalization procedure showed a left common femoral vein access with occluded left external iliac vein and mature pelvic collaterals draining to the right external iliac vein.
7.2.5 Specifics of Consent
The risks and benefits of reattempting recanalization of the patient’s chronically occluded left external iliac vein were discussed in detail. Specifically, the expectation of getting through a chronic occlusion that had been previously attempted was put into perspective. Potential complications such as vascular injury and bleeding were discussed. Complications such as iliac vein thrombosis or stent occlusion that could necessitate additional procedures in the future were also described. Self-limited side effects, including access site pain and back pain, that may accompany iliac vein stenting were discussed. Although the likelihood of using thrombolytics was low due to the chronicity of the occlusion, the risks of bleeding from thrombolytics was discussed if acute or subacute thrombus was found during the procedure requiring pharmacomechanical thrombectomy. Finally, a tentative plan for postprocedural anticoagulation was outlined, including the type and length of anticoagulation.
Details of Procedure
A preprocedural plan was formulated to ensure the best possible outcome. The plan was to gain access to the left femoral vein in the midthigh or the left great saphenous vein with an 8F, 10-cm sheath and perform a venogram. Attempts would be made to cross the occluded left external iliac vein with a hydrophilic guidewire, 5F Bern catheter (Boston Scientific, Marlborough, MA), followed by angioplasty and Wallstent placement (Boston Scientific, Marlborough, MA). Intravascular US (IVUS) (Volcano Corporation, San Diego, CA) would also be used for sizing and stent positioning and to determine the extent of compression of the left common iliac vein.
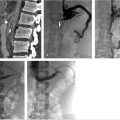
The patient was placed in the supine position, general anesthesia induced, a bladder catheter placed, and US evaluation performed to determine the optimum access site. The right neck and left groin down to the knee were prepped. Initially access was gained in the left greater saphenous vein should stenting be required into the femoral vein. Venography was performed which showed occlusion of the left common iliac vein with patent left internal iliac draining to the iliac confluence and cross-pelvic collaterals draining to the right external iliac vein (Fig. 7.3a). Second access was obtained in the right internal jugular vein and a long 6F braided sheath was placed to the level of the left common iliac occlusion (Fig. 7.3b). Several attempts were made with a 5F Bern catheter and stiff hydrophilic wire to cross the occluded left common iliac vein without success.
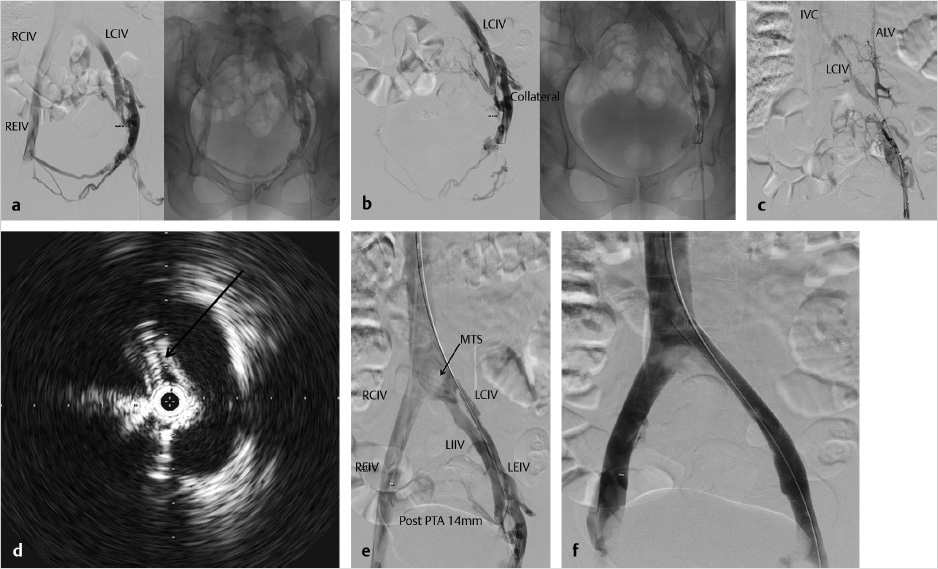
Venography was performed in different obliquities in an attempt to find a channel that resembled the occluded left external iliac vein. Oblique femoral venography showed a very diminutive venous collateral representing the ascending lumbar vein, a branch of the left common iliac vein (Fig. 7.3c). Accessing the channel to this venous branch, a 5F Quick-cross catheter (Spectranetics, Colorado Springs, CO) was used with a hydrophilic wire to traverse the occluded left common iliac vein. Angioplasty was performed with a 6-mm balloon after which IVUS was used to determine the extent of compression and facilitate stent sizing (Fig. 7.3d). Serial angioplasty was performed up to 16 mm (Fig. 7.3e).
The length of the common iliac and external iliac vein requiring stenting was approximately 16 cm. An 18 mm × 9 cm Wallstent was placed in the left common iliac vein with 1 cm extending into the IVC and was balloon dilated to 16 mm, resulting in a total stent length of 10 cm. A second 16 mm × 9 cm Wallstent with 1 cm of overlap into the left common iliac stent was placed in the left external iliac vein down to but not past the inguinal ligament and ballooned to 16 mm (Fig. 7.3f). IVUS was repeated and showed significant improvement in iliac vein diameter, completion venography was performed, and hemostasis was achieved with manual pressure.
There were no postprocedure complications. The patient was extubated, the bladder catheter removed, and the patient transferred to recovery. The patient was admitted to ensure that there were no postprocedure complications; pain was adequately controlled, and anticoagulation was appropriately administered.
7.2.6 Follow-up
At a 3-week postprocedure clinic visit, symptoms of CVOD had resolved and she had returned to her normal activity including long-distance running.
7.3 Case Vignette 2
7.3.1 Patient Presentation
A 61-year-old male was referred 3 days after undergoing coronary artery bypass grafting complicated by acute IVC and bilateral iliac vein thrombosis. The patient had been on warfarin for a right lower extremity DVT and, in preparation for surgery, had a Trapease IVC filter (Cardinal Health, Dublin, OH) placed 3 weeks prior to surgery. After examination of the patient and extensive discussions with the cardiac surgery team at our institution, the decision was made to discharge the patient on anticoagulation and compression stocking therapy with follow-up evaluation in interventional radiology (IR) clinic in 4 to 6 weeks, as it was determined that thrombolytic therapy would not be safe in the immediate post–cardiac surgery setting.
One month later, the patient presented to IR outpatient clinic for evaluation. The symptoms of bilateral lower extremity swelling had minimally improved with anticoagulation and stockings. He continued to have significant lower extremity pain and swelling and was unable to stand or ambulate for long periods of time. He had gained nearly 25 lbs from lower extremity edema. The patient felt that his cardiac rehabilitation was compromised due to his symptoms resulting from extensive lower extremity clot burden.
7.3.2 Physical Exam
On physical examination, the patient had significant bilateral lower extremity swelling. His calves were tender to palpation and he was unable to stand for more than a few minutes without developing lower extremity pain and fatigue. He had no obvious skin manifestations, varicosities, or ulcers.
7.3.3 Imaging
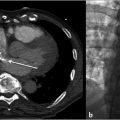
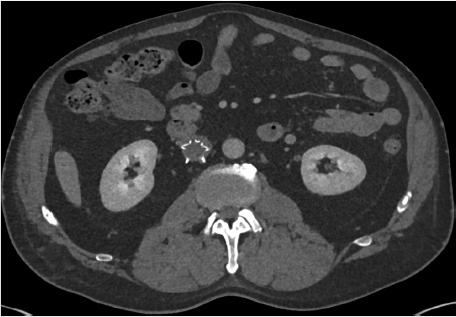
A CT venogram demonstrated the permanent IVC filter with occlusive thrombosis of the infrarenal IVC and bilateral iliac veins (Fig. 7.4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree