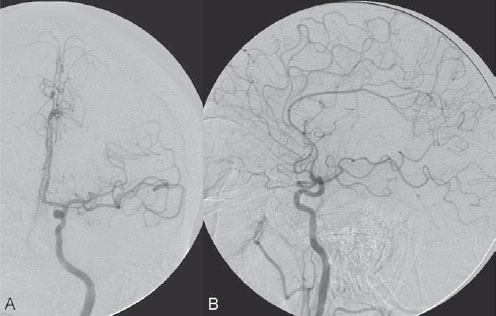
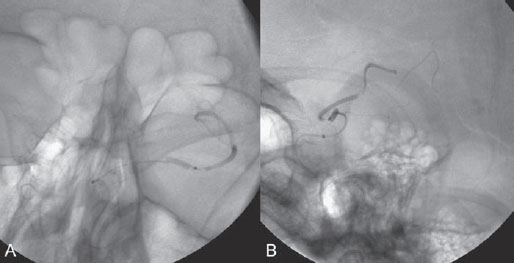
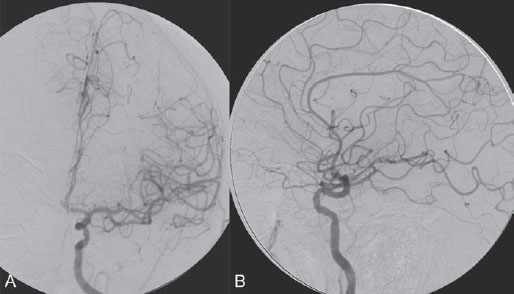
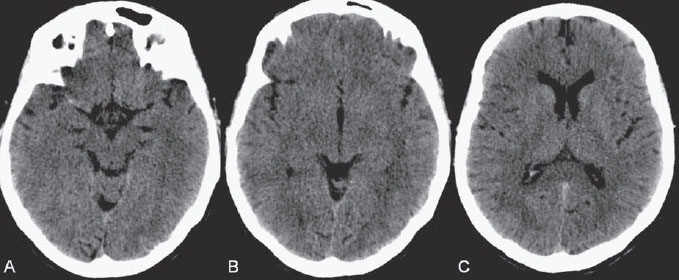
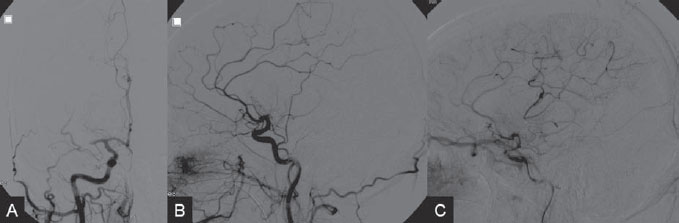
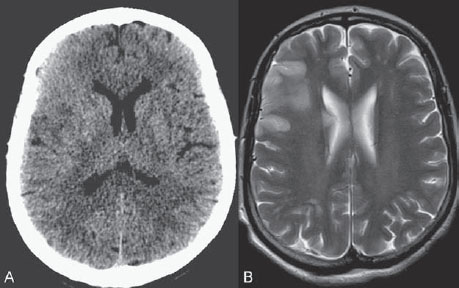
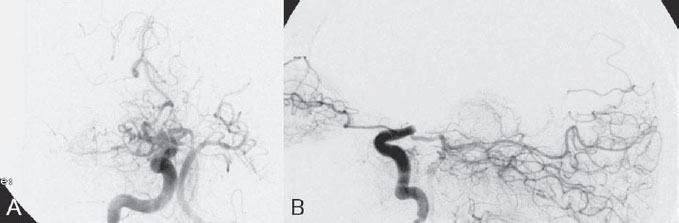
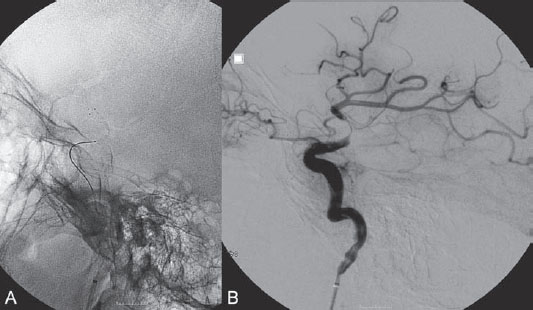
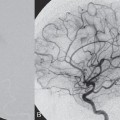
PART VII Stroke A 68-year-old previously healthy woman experiences the acute onset of aphasia and right-sided hemiplegia. She is brought to the emergency department within the first hour after symptom onset, and emergency CT is performed. Fig. 47.1 DSA. Left ICA angiogram in (A) AP and (B) lateral views demonstrates occlusion of the proximal middle branch of the left MCA. CT CT was unremarkable and did not demonstrate any signs of acute stroke, especially no hyperdense middle cerebral artery (MCA) sign. CTA demonstrated an M2 occlusion on the left side. Acute left-sided MCA branch occlusion EQUIPMENT DESCRIPTION After the left common carotid artery had been selected, a roadmap was performed to exclude the possibility of a high-grade stenosis of the internal carotid artery (ICA) origin, and the catheter was advanced via the guidewire into the left ICA. Angiography demonstrated an open M1 but a complete occlusion of the dominant middle trunk of the MCA trifurcation, which left a large perfusion deficit within the left hemisphere (Fig. 47.1). A bolus of 2000 units of heparin was given, and the heparin was continued with the injection of 450 units per hour. Following this, a standard Excel 14 micro-catheter equipped with a Transend 14 Soft Tip was advanced via the guiding catheter, which was continuously flushed with heparinized saline. The microcatheter was also continuously flushed and advanced with caution under roadmap conditions distal to the thrombus into the distal M2 branch, as verified by a gentle microcatheter injection (Fig. 47.2). Subsequently, 1 mg of recombinant tissue plasminogen activator (rtPA) dissolved in 2 mL of saline was injected distal to the clot over 1 minute. The microcatheter was then slowly withdrawn into the proximal part of the thrombus, where another bolus of 1 mg of rtPA was administered. A continuous injection of 10 mg of rtPA per hour was performed at this point (proximal part of the thrombus). Control runs via the guiding catheter were performed every 15 minutes. After 1 hour and the administration of a total of 12 mg of rtPA intra-arterially, there was still no change visible at catheter angiography, with complete occlusion of the proximal M2 branch. After the administration of another 2.5 mg of rtPA, however, the control run demonstrated complete reopening of the distal and proximal M2 branch, with minimal stagnation of contrast in distal M4 branches. The microcatheter was removed, and the final control run demonstrated complete reopening of the M2 branch with reperfusion of more than 90% of its territory (Fig. 47.3). The guiding catheter was removed, and the sheath was sutured to the groin. During a neurologic examination on the table, the patient was able to speak again, and the hemiplegia had completely resolved. Follow-up CT 1 day later demonstrated no area of ischemia. Fig. 47.2 Unsubtracted gentle microcatheter injection in (A) AP and (B) lateral views reveals a filling defect, representing thrombus in the MCA branch. Fig. 47.3 DSA. Left ICA angiogram in (A) AP and (B) lateral views after successful thrombolysis shows complete reopening of the entire MCA. Stroke is one of the leading causes of death or long-term disability, and demographic changes are likely to result in an increase in both the incidence and prevalence of stroke. Stroke is a common cause of dementia, epilepsy, and depression in the elderly. More than 85% of all strokes are ischemic in nature. Up to 30 to 35% of ischemic strokes are due to extracranial or intracranial large-artery atherosclerosis. Cardioembolic strokes account for 25 to 30% of all cerebral ischemic events. Small-vessel disease accounts for 15 to 20% of all ischemic strokes. The remaining ischemic strokes have either another, uncommon cause or an undetermined origin. Nearly 10% of patients with acute stroke die, and of those who survive, about 50% are disabled. Older age, a poor neurologic score on admission, high blood levels of glucose, and a large clot burden increase the chance of a poor outcome. Ischemic stroke is caused by a critical reduction in the blood supply to the brain for a prolonged period. Following the occlusion of a brain-supplying artery, a core of infarcted tissue will develop, surrounded by tissue that is initially only functionally impaired because of a reduced blood supply. The amount of functionally impaired tissue depends on the location of the occluded vessel and the collateral blood supply. Reperfusion can salvage those cells at risk for critical hypoperfusion. In a recent Cochrane Database Review of more than 7000 patients, Wardlaw et al stated that thrombolytic therapy, mostly administered up to 6 hours after ischemic stroke, significantly reduced the proportion of patients who died or became dependent (modified Rankin Scale score of 3–6) at 3 to 6 months after stroke (odds ratio [OR], 0.81;95% confidence interval [CI], 0.73–0.90). However, the route of administration remains controversial (i.e., intravenous, intra-arterial, or bridging therapy, which is started with the intravenous administration and continued with the intra-arterial administration of a drug). PHYSICAL EXAMINATION CT/MRI CONSERVATIVE OR MEDICAL MANAGEMENT ENDOVASCULAR TREATMENT Published Literature on Treatment Options It is beyond doubt that longer periods of time between the onset of symptoms of ischemic stroke and the initiation of treatment are associated with a progressively lower likelihood of clinical benefit. Likewise, the probability of a good clinical outcome decreases as the time to angiographic reperfusion increases. However, the way to reach the goal of rapid recanalization remains controversial. Physicians who favor the use of intravenous therapy alone base their arguments mostly on the fact that it is the only FDA-approved therapy for improving the outcome of acute stroke. Data to support this came from the National Institute of Neurological Disorders and Stroke trial of intravenous thrombolysis conducted in 1995. Intravenous rtPA was given in a dose of 0.9 mg/kg of body weight, with a maximum dose of 90 mg, within the first 3 hours after symptom onset. Compared with patients in the placebo arm of the study, patients in the treatment arm were 30% more likely to have minimal or no disability at 90 days. The rate of symptomatic intracranial hemorrhage was higher in those receiving treatment (6.4%) than in the controls (0.6%). However, the mortality rates at 90 days were similar. The recent European Cooperative Acute Stroke Study (ECASS III), a randomized, double-blind, multicenter, placebo-controlled trial of rtPA in acute ischemic stroke in which thrombolysis was initiated between 3 and 4.5 hours after stroke onset, extended even further the time window in which the intravenous administration of rtPA was proven beneficial. The arguments against intra-arterial therapies that are often put forward are the greater complexity of the procedure, lack of availability, potential delays in initiating treatment, added risks of an invasive procedure, and higher expenses. In intra-arterial thrombolysis, the drug is given only to those patients who have an occluded artery; the dosage to be given is usually smaller and may therefore be safer. Further data in favor of endovascular therapies are derived from the Prolyse in Acute Cerebral Thromboembolism (PROACT) trials, which demonstrated good outcomes for patients with large-vessel occlusion given intra-arterial therapy. A significant 15% increase in the chance of a good outcome was noted when patients with M1 occlusion were treated with intra-arterial thrombolysis within a 6-hour time window without an increase in mortality in comparison with those who did not receive this intervention. In a recent observational study that compared data from two stroke units where the management of stroke was similar, except that one unit performed intra-arterial thrombolysis with urokinase and the other intravenous thrombolysis with plasminogen activator, intra-arterial thrombolysis was more beneficial than intravenous thrombolysis, even though intra-arterial thrombolysis was started later. A bridging strategy between intravenous and intra-arterial thrombolysis has the advantage of not delaying lytic therapy while identifying nonresponders with persistent large-artery occlusion. Initial positive results for this treatment strategy have been reported by the RECANALISE study, which compared recanalization rates, neurologic improvement, and functional outcome in patients treated within the first 3 hours after stroke onset either with intravenous thrombolysis alone or with a combined intravenous-endovascular approach. The authors found that an intravenous-endovascular approach was associated with higher recanalization rates than intravenous alteplase in patients with stroke and confirmed arterial occlusion. The clinical outcome was more often favorable in the intravenous-endovascular group, whereas mortality rates and rates of hemorrhagic stroke were similar in the two groups. The bridging approach is currently being tested in the Interventional Management of Stroke (IMS III) trial, in which initial intravenous tPA is followed by artery reopening with thrombolytic agents or clot retrieval if vessel occlusion is demonstrated. According to the presently available data, intravenous thrombolysis within the first 3 hours after stroke onset will result in less than a 30% chance of reopening large arteries with a large clot burden, versus a 60% to 70% chance of recanalization with intra-arterial thrombolysis. Given these data, one may be inclined to reassess current management strategies for patients with acute stroke in the 3-hour time period. If a patient has a major large-vessel occlusion and considerable mismatch, intra-arterial treatment may be considered. If significant delays in starting intra-arterial treatment are anticipated, intravenous thrombolysis can be initiated, followed by intra-arterial treatment if recanalization does not occur. For other cases in the early time window, intravenous thrombolysis may still be considered. After the 3- (or 4.5-) hour time window, treatment should be guided by the results of the PROACT II trial, which indicated a better outcome for patients treated with intra-arterial thrombolysis within up to 6 hours after stroke onset. PEARLS AND PITFALLS__________________________________________________ Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study:a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282(21):2003–2011 Jahan R, Vinuela F. Treatment of acute ischemic stroke: intravenous and endovascular therapies. Expert Rev Cardiovasc Ther 2009;7(4):375–387 Mattle HP, Arnold M, Georgiadis D, et al. Comparison of intraarterial and intravenous thrombolysis for ischemic stroke with hyperdense middle cerebral artery sign. Stroke 2008;39(2):379–383 Mazighi M, Serfaty JM, Labreuche J, et al; RECANALISE investigators. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol 2009;8(9):802–809 Schonewille WJ, Wijman CA, Michel P, et al; BASICS study group. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol 2009;8(8):724–730 Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333(24):1581–1587 Wardlaw JM, Murray V, Berge E, Del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2009;(4):CD000213 A 68-year-old woman presents to the emergency department 5 hours after the sudden onset of left hemiparesis. Her baseline National Institutes of Health Stroke Scale (NIHSS) score is 19. Risk factors include chronic hypertension and heavy smoking. Emergency CT, including CTA and CT perfusion, is performed. Fig. 48.1 (A) Unenhanced axial CT scan demonstrates a hyperdense right MCA sign and (B,C) slight hypodensity within the right insular and fronto-opercular regions. CT Plain CT of the brain demonstrated a hyperdense right middle cerebral artery (MCA) sign and mild right insular and fronto-opercular hypodensity in less than one-third of the MCA territory. CTA confirmed occlusion of the distal M1 and corresponding hypoperfusion (Fig. 48.1). DSA The common carotid artery (CCA) and the internal carotid artery (ICA) origins were unremarkable. Intracranially, there was a right MCA occlusion. During late arterial phases, mild pial collateral filling of the distal MCA via the anterior cerebral artery (ACA) was seen (Fig. 48.2). MCA occlusion at the M1 (Thrombolysis in Cerebral Infarction [TICI] grade, 0). EQUIPMENT DESCRIPTION Following diagnostic angiography, an 8F multipurpose catheter was advanced into the right ICA. A Penumbra Aspiration Microcatheter 041 with a 30-degree angled curve was introduced with a 0.016-in guidewire and advanced gently to the MCA proximal to the clot under roadmap guidance. The guidewire was removed, and the Penumbra Separator 041 was advanced. The connection to the pump was opened at a pressure of −20 mm Hg. When refluxed blood started to appear in the connecting tubes to the pump, the separator was advanced and used to disperse the thrombus while the microcatheter was slowly advanced. An intermediate control demonstrated a persistent M2 branch occlusion and some distal emboli in frontal branches. A solution of recombinant tissue plasminogen activator (rtPA) was then infused through the microcatheter, which was positioned at the M1. Progressive reperfusion was observed during infusion of a total of 12 mg until a TICI grade of 2b was achieved (Fig. 48.3). After the procedure, the patient was taken to the intensive care unit and extubated. A small residual motor deficit of the left arm was noted. At 24 hours, the NIHSS score was 2. The patient recovered completely and was discharged at day 7 (Fig. 48.4). Fig. 48.4 (A) Unenhanced axial CT scan at 24 hours after treatment reveals no evidence of intracranial hemorrhage. There is mild effacement of the cortical sulci in the right fronto-opercular region. (B) Axial T2-weighted MR image 3 days after treatment shows a small right frontal infarction. As stated in the previous case, ischemic stroke is one of the leading causes of disability and mortality in the world. Intravenous thrombolysis is at present the only approved treatment of this devastating disease, with the therapeutic window extended to 4.5 hours after symptom onset. With intravenous thrombolysis, however, recanalization rates in patients with proximal large-vessel occlusions are low; reported rates are 10% for the ICA and 30% for the proximal MCA. In addition, more than 80% of patients with an NIHSS score of 10 or higher have persistent arterial occlusion on subsequent angiography, even after initial treatment with intravenous tPA. Therefore, there is keen interest in intraarterial therapies for acute stroke that may extend the therapeutic window and make it possible to treat patients who have contraindications to or who have failed intravenous thrombolysis. Advantages of intra-arterial thrombolysis are higher rates of recanalization, smaller doses of rtPA, and immediate confirmation of recanalization;disadvantages are the limited availability of this technique in the community and the slightly higher rate of hemorrhagic complications. Given the risk for hemorrhage and the time constraints associated with thrombolytic agents, alternate means for removing a clot have been proposed that are discussed here and in the next case. PHYSICAL EXAMINATION CT MEDICAL OR SURGICAL TREATMENT ENDOVASCULAR TREATMENT
| Category | Results | Score |
| 1a. LOC (Patients with a score of 2 or 3 for this item should be assessed with the Glasgow Coma Scale.) | Alert, keenly responsive Not alert (arousable–minor stimulation) Not alert (arousable–painful stimulation) Unresponsive | 0 1 2 3 |
| 1b. LOC questions (month, age) | Answers both correctly Answers one correctly Answers neither correctly | 0 1 2 |
| 1c. LOC commands (open and close eyes, make and release first) | Performs both tasks correctly Performs one correctly Performs neither correctly | 0 1 2 |
| 2. Best gaze | Normal Partial gaze palsy Forced deviation | 0 1 2 |
| 3. Visual | No visual loss Partial hemianopia Complete hemianopia Bilateral hemianopia | 0 1 2 3 4 |
| 4. Facial palsy | Normal Minor paralysis Partial paralysis Complete paralysis | 0 1 2 3 |
| 5. Motor function (arm) a. Left b. Right | No drift Drift before 5 seconds Drift before 10 seconds No effort against gravity No movement | 0 1 2 3 4 |
| 6. Motor function (leg) a.left b.left | No drift Drift before 5 seconds Drift before 10 seconds No effort against gravity No movement | 0 1 2 3 4 |
| 7. Limb ataxia | No ataxia Ataxia–one limb Ataxia–two limbs | 0 1 2 |
| 8. Sensory function | No sensory loss Mild sensory loss Severe sensory loss | 0 1 2 |
| 9. Language | Normal Mild aphasia Severe aphasia Mute or global aphasia | 0 1 2 3 |
| 10. Articulation | Normal Mild-to-moderate dysarthria Severe dysarthria | 0 1 2 |
| 11. Extinction and inattention | Absent Mild Severe | 0 1 2 |
Abbreviation: LOC, level of consciousness
| Post-treatment TICI Reperfusion Grade | |
| 0 | No perfusion |
| 1 | Perfusion past the initial obstruction but limited distal branch filling with little or slow distal perfusion |
| 2a | Perfusion of less than half of the vascular distribution of the occluded artery (e.g., filling and perfusion through one M2 division) |
| 2b | Perfusion of half or more of the vascular distribution of the occluded artery (e.g., filling and perfusion through two or more M2 divisions) |
| 3 | Full perfusion with filling of all distal branches |
| Post-treatment AOL Recanalization Grade | |
| 0 | No recanalization of the occlusion |
| 1 | Incomplete or partial recanalization of the occlusion, with no distal flow |
| 2 | Incomplete or partial recanalization of the occlusion, with any distal flow |
| 3 | Complete recanalization of the occlusion with any distal flow |
Abbreviations: AOL, arterial occlusive lesion; TICI, Thrombolysis in Cerebral Infarction
- Thrombolytic therapy can be used primarily to dissolve the clot or at the end of a mechanical procedure to treat distal emboli. Although there is no consensus in the literature about what constitutes a safe dose, most authors quote a maximum of 22 mg of intra-arterial rtPA within the first 6 hours after stroke onset.
- Distal emboli (which can be treated with further intra-arterial injection of rtPA)
- Intracranial vascular dissection, subarachnoid hemorrhage, and vessel rupture
- Reperfusion syndrome and hemorrhagic transformation of acute stroke
Published Literature on Treatment Options
The goal of recanalization therapy in acute ischemic stroke is to improve the clinical outcome by restoring antegrade perfusion and salvaging brain tissue at risk. Early vessel recanalization is linked to a good clinical outcome. Mechanical clot removal is an alternative to intravenous or intra-arterial medical thrombolysis, especially in patients with failed medical treatment, those with contraindications to rtPA (e.g., recent surgery), and those with a large clot burden. In this case, we focus on the two devices that are currently approved by the FDA;in the next case, we discuss additional new and promising devices for the mechanical treatment of stroke.
The MERCI device consists of a flexible, tapered nitinol wire with five helical loops that can be embedded within the thrombus for retrieval. Two major trials, Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI, have demonstrated its usefulness, especially in large-vessel occlusions. In these two trials, 48 to 68% of occluded vessels were recanalized, with a 5 to 7% rate of clinically significant procedural complications, an 8 to 10% rate of symptomatic hemorrhage, and a 34 to 44% mortality rate.
The Penumbra System is specifically designed to remove thrombus in large intracranial vessels. Revascularization with thrombus debulking and aspiration is followed by direct extraction of thrombus if clot remains. Once the catheter is positioned just proximal to the clot, the aspiration pump is connected to the catheter, and a vacuum of –20 mm Hg is produced that will facilitate aspiration of the clot. The separator is then advanced through the catheter into the proximal part of the clot, aiding the aspirating and debulking process, until the distal aspect of the clot is reached. In a preliminary clinical trial, recanalization was achieved in 82% of occluded vessels, with a 3% rate of clinically significant complications, an 11% rate of symptomatic hemorrhage, and a mortality rate of 33%.
The present trials do not have a nontreatment arm and so cannot directly demonstrate the superiority of mechanical treatment versus no treatment or medical treatment. However, the rate of artery recanalization far exceeds the expected rate of spontaneous or drug-induced early recanalization. Therefore, mechanical thrombectomy for acute stroke is likely to be beneficial in appropriate patients.
PEARLS AND PITFALLS__________________________________________________
- An intra-arterial procedure after intravenous thrombolysis requires careful attention to the puncture site. A one-wall technique or a micro-puncture set can be used. Heparin is not indicated when a previous dose of rtPA has been given because it increases the risk for hemorrhage.
- For aspiration procedures, the following should be noted:
- Larger catheters work better and faster (Penumbra 041 and 054).
- Do not allow the aspiration system to be blocked for a long time. Continuous reflux should be maintained to avoid the formation of clot inside the catheter system, which decreases the effcacy of aspiration or permanently blocks the catheter.
- Always start aspirating at the proximal aspect of the clot, precisely at the zone between the clot and the vessel to be opened.
- The separator is used to break up the clot, so that it enters the microcatheter more easily. It should never be used as a guidewire.
- If the tip of the microcatheter is inside the thrombus and aspiration suddenly stops, the separator should be kept in position while the microcatheter is pulled back until reflux starts again.
- For distal emboli, intra-arterial rtPA can be administered through the microcatheter or guiding catheter.
- Larger catheters work better and faster (Penumbra 041 and 054).
Further Reading
Gandhi CD, Christiano LD, Prestigiacomo CJ. Endovascular management of acute ischemic stroke. Neurosurg Focus 2009;26(3):E2
Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS; MERCI; Multi MERCI Writing Committee. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke 2009;40(12):3777–3783
Nogueira RG, Schwamm LH, Hirsch JA. Endovascular approaches to acute stroke, part 1: Drugs, devices,and data. AJNR Am J Neuroradiol 2009;30(4):649–661
Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 2009;40(8):2761–2768
Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001;22(8):1534–1542
Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome:a meta-analysis. Stroke 2007;38(3):967–973
Tomsick T, Broderick J, Carrozella J, et al ; Interventional Management of Stroke II Investigators. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol 2008;29(3):582–587
A 48-year-old man with a known patent foramen ovale presents to the emergency department with left hemiparesis 2 hours after symptom onset. His baseline National Institutes of Health Stroke Scale (NIHSS) score is 12. Emergency CT is performed.
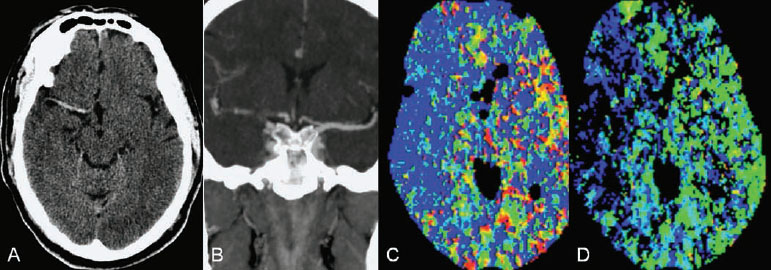
Fig. 49.1 (A) Unenhanced axial CT scan demonstrates a hyperdense right MCA sign and no evidence of hypodensity or intracranial hemorrhage. (B) CTA in coronal view shows occlusion of the right ICA bifurcation and the right MCA. (C,D) CT perfusion reveals hypoperfusion of the right MCA territory (blue).
CT
Plain CT of the brain demonstrated no evidence of hypodensity (Alberta Stroke Program Early CT Score [ASPECTS] of 10), but a dense right middle cerebral artery (MCA) was noted. CTA and CT perfusion demonstrated an occlusion of the right internal carotid artery (ICA) bifurcation, occlusion of the MCA, and hypoperfusion of the right MCA territory (Fig. 49.1).
DSA
The patient received 0.6 mg of recombinant tissue plasminogen activator (rtPA) per kilogram of body weight intravenously over 30 minutes and was re-evaluated clinically and with transcranial Doppler ultrasound. His clinical status was unchanged, and the transcranial Doppler study showed complete occlusion of the right MCA (Thrombolysis in Cerebral Infarction [TICI] grade, 0). Intra-arterial treatment was initiated.
Injection of the right ICA showed a carotid T occlusion with the clot burden starting beyond the posterior communicating artery. Reflux via the posterior communicating artery into the basilar artery was noted (Fig. 49.2).
Acute occlusion of the carotid termination
EQUIPMENT
- Standard 8F access (puncture needle, 8F vascular sheath)
- Standard 8F balloon guide catheter (Concentric Medical, Mountain View, CA) with continuous flush and a 0.035-in hydrophilic guidewire (Terumo, Somerset, NJ)
- Preparation for acute stenting procedure
- Rebar 18 Micro Catheter (ev3, Plymouth, MN), curved at vapor (30 degrees) with continuous flush
- A 0.014-in hydrophilic micro-guidewire (Synchro2 14;Boston Scientific, Natick, MA)
- Solitaire FR Revascularization Device 4 × 20 mm (ev3)
- Rebar 18 Micro Catheter (ev3, Plymouth, MN), curved at vapor (30 degrees) with continuous flush
- Contrast material
- Recombinant tissue plasminogen activator (Actilyse;Genentech, South San Francisco, CA) 1-mg/mL solution
DESCRIPTION
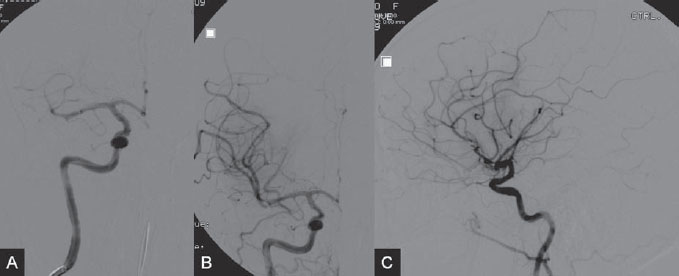
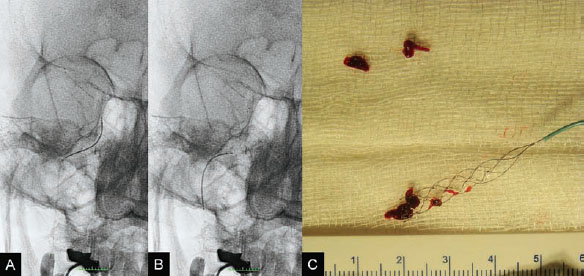
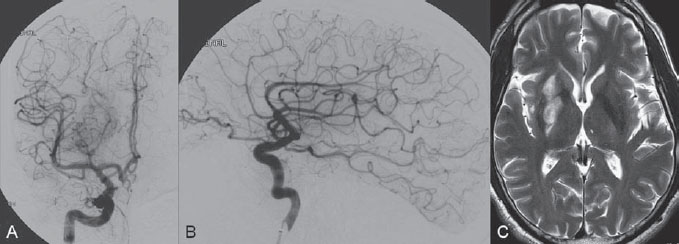
The patient was taken to the angiography suite and put under general anesthesia. Based on the vascular information obtained from the angiographic CT, complete diagnostic angiography was not performed. The 8F, 90-cm balloon guide catheter was placed directly into the right ICA. Under fluoroscopic control, an 18-in microcatheter was manipulated over a 14-in micro-guidewire across the occlusion site until it was distal to the thrombus. A 4 × 20-mm Solitaire FR Revascularization Device was deployed across the thrombus, and an angiographic control run was performed. Immediate reperfusion (TICI grade, 1) was achieved on deployment of the Solitaire FR Device, which was followed by the intra-arterial infusion of 5.0 mg of rtPA for 5 minutes through the balloon guide catheter (Fig. 49.3). Because the clot was not dissolving with the proximal injection of rtPA, the next step was to use the Solitaire FR Device as a retriever. The device was recovered during carotid occlusion with the balloon guide catheter and continuous aspiration from the Y connector of the guiding catheter to remove the parts of the clot that could be detached from the device. The procedure was repeated twice, resulting in complete removal of the residual clot (Fig. 49.4). The final control angiogram showed complete recanalization of the MCA, and follow-up MRI 3 days after the procedure demonstrated a small residual infarction at the right basal ganglion (Fig. 49.5). The patient was discharged from the hospital and was able to return to his normal activities with rehabilitation.
Fig. 49.4 (A,B) Plain radiography in AP view during recovery of the device. Note inflation of the balloon guide catheter to stop flow in the ICA during recovery of the device. (C) The recovered device and retrieved clot.
Fig. 49.5 DSA. Right ICA angiogram in (A) AP and (B) lateral views after the procedure shows complete recanalization (TICI grade of 3). (C) Axial T2-weighted MR image 3 days after the procedure demonstrates a small residual infarction at the right basal ganglion.
In the treatment of acute stroke, the major concerns at present are the limited treatment window, the risk for hemorrhage associated with intravenous and intra-arterial thrombolytic agents, and the disappointing rates of recanalization reported with mechanical embolectomy. These shortcomings, together with the successful application of angioplasty and stenting in acute cardiac ischemia, have prompted the application of intracranial angioplasty and stenting in the acute phase of cerebral ischemia. However, caution must be exercised?because the vasculature and circulation of the cardiac system differ significantly from those of the cerebrovascular system. In the present case, a relatively new device is described that has demonstrated good recanalization rates without being overly aggressive.
TRANSCRANIAL DOPPLER ULTRASOUND
- Together with the clinical evaluation, transcranial Doppler ultrasound is a helpful tool to determine noninvasively at the bedside whether a vessel has reopened following the intravenous administration of rtPA.
CT/MRI
- Unenhanced CT is at present the most widely used screening tool for patients with acute stroke. However, modern MRI and CT techniques (i.e., diffusion- and perfusion-weighted imaging and perfusion CT) have been introduced that allow a comprehensive, noninvasive survey of patients with acute stroke. They accurately demonstrate the site of arterial occlusion and its hemodynamic and pathophysiologic effects on the brain parenchyma. After the onset of ischemic stroke, potentially viable tissue may be salvageable for as long as 48 hours. A combination of diffusion-weighted and perfusion-weighted MRI or perfusion CT may improve the selection of patients with potentially salvageable tissue and should therefore be used as soon as patients arrive in the emergency department.
ENDOVASCULAR TREATMENT
- Current reperfusion strategies are many, and some are still in the investigational phase. The following strategies are available.
- Endovascular thrombectomy with distal devices:MERCI (Concentric), CATCH (Balt), Phenox clot retriever (Phenox), Neuronet (Guidant Endovascular), Attractor 18 (Target Therapeutics); endovascular thrombectomy with proximal devices:Alligator (ev3), InTime Retriever (Boston Scientific), snares
- Endovascular mechanical aspiration with large catheters (Penumbra stroke system, Possis AngioJet) that have been shown to be effective in patients with a large clot burden (carotid T and M1 portion of the MCA)
- Mechanical disruption of thrombus with micro-guidewires, snares, or balloon angioplasty
- Transcranial augmented fibrinolysis (transcranial Dopper ultrasound) or endovascular augmented fibrinolysis (EKOS)
- Endovascular entrapment of thrombus with balloon-expandable stents
- Temporary endovascular bypass with resheathable stents
- Endovascular thrombectomy with distal devices:MERCI (Concentric), CATCH (Balt), Phenox clot retriever (Phenox), Neuronet (Guidant Endovascular), Attractor 18 (Target Therapeutics); endovascular thrombectomy with proximal devices:Alligator (ev3), InTime Retriever (Boston Scientific), snares
- Distal embolic events, intracranial vascular dissection, rupture with subarachnoid hemorrhage
- Reperfusion syndrome and hemorrhagic transformation of acute stroke
Published Literature on Treatment Options
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree