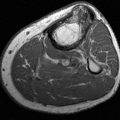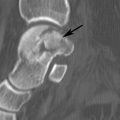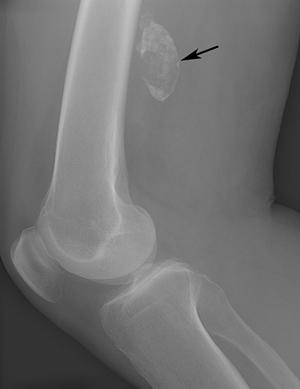
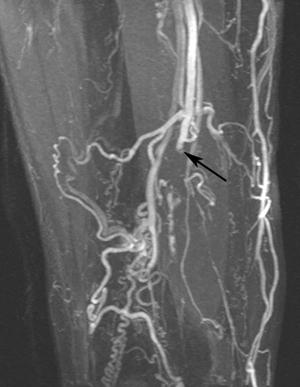
FIGURE 8-27 Calcified thrombosed aneurysm. AP (A) and lateral (B) radiographs demonstrate peripheral calcification in the aneurysm (arrow).C) MR angiogram demonstrates complete occlusion (arrow) at the level of the aneurysm with extensive collaterals.
Radioisotope Imaging
Today, radionuclide studies are less frequently used to study flow and healing potential in ischemia of the foot and ankle [83,195]. In the past, technetium-99m, indium-111, xenon-133, and thallium-201 have all been evaluated as noninvasive imaging techniques [132,159,167]. Lawrance et al. [132] found that three-phase technetium scans were useful in assessing local perfusion and predicting healing of ischemic ulcers. All ulcers studied healed when activity was increased in the region of the ulcer compared with normal tissue background or the normal extremity. Healing was uncommon when uptake was not increased in the ulcerated areas.
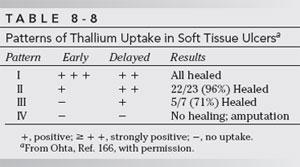
Thallium-201 has been used for similar purposes [167,208]. Ohta [167] compared patterns of thallium-201 uptake (a marker of perfusion and tissue viability) in healing and nonhealing ulcers in 36 patients. The pattern of isotope uptake was correlated with hyperemia and inflammation in the ulcer bed. Both early (5 to 15 minutes) and delayed (3 hours) images were obtained after 2 mCi of thallium-201 was given intravenously. Four patterns of uptake were described (Table 8-8). All patients with no increase in uptake on either early or delayed studies eventually required amputation [167]. Siegel et al. [208] found thallium-201 comparable to Doppler techniques in predicting healing of cutaneous ulcerations.
Platelets labeled with indium-111 have also been employed in studies of patients with soft tissue ischemia. Comerford et al. [40] studied 20 patients using this technique. They noted reduced sensitivity from false-negative studies but found indium-111–labeled platelets useful in predicting healing. In addition, by combining indium-111 with technetium-99m three-phase studies, the results were more accurate. Intradermal xenon-133 injections have also been used to assess cutaneous flow [132]. This technique is more difficult to evaluate and causes more patient discomfort. Thallium-201 studies are accurate but are much more expensive than conventional three-phase technetium studies [132,144].
Radionuclide studies can be useful, but spatial resolution is limited, especially in the lower leg [83]. Therefore, other techniques, such as ultrasound, CT, or MR angiography or convention angiography are more often used at most centers.
Ultrasound
Ultrasonographic techniques provide a simple, noninvasive method of assessing peripheral flow in patients with peripheral arterial disease [6,18,20,41,63,83,124,128,129,179]. Today, color flow duplex sonography and power Doppler techniques provide improved sensitivity and specificity for evaluation of peripheral arteries. Doppler ultrasound is particularly useful in patients with ischemic disease, diminished peripheral pulses, and reduced peripheral systolic pressures (less than 70 mm Hg) [41]. Advantages of ultrasound include its low cost, versatility (can be used at the bedside), ease of use, and ability to differentiate occlusive from nonocclusive vascular disease [18,20,63,83,179]. The normal Doppler arterial wave form in the peripheral arteries is triphasic [20,179]. During systole, a fast forward pattern is followed by brief period of flow reversal during early diastole followed by the third phase of forward flow in late diastole (Fig. 8-28) [20]. The second phase (flow reversal) is absent in the presence of significant arterial stenosis. Distal to occlusions, the pulse is monophasic [128]. The reversal portion of the pattern may also be absent in diabetic patients. In the presence of neuropathy, the flow may be increased up to five times [16,41,128]. This flow abnormality is most likely related to peripheral autonomic neuropathy with dilatation of the vascular bed [41,129].
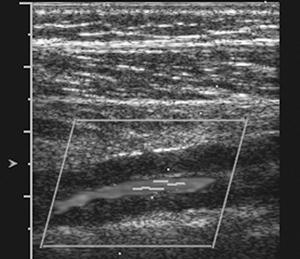
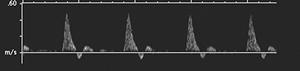
FIGURE 8-28 Normal posterior tibial artery (A) with triphasic wave form (B).
Stenosis can be evaluated using changes in the wave form and peak systolic velocity. Stenoses less than 20% may result in changes in the wave form, but peak systolic velocities do not change significantly. Stenoses between 20% and 49% demonstrate a 30% to 100% increase in peak velocity. More significant stenosis (greater than 50%) demonstrate greater than 100% increase in peak velocity compared to the normal prestenotic segment with loss of the flow reversal phase (Fig. 8-29) [20]. In the presence of long narrowed segments (greater than 5 cm), the peak velocity may decrease [179]. There is no Doppler signal with complete occlusion [179].
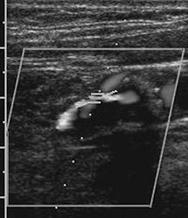
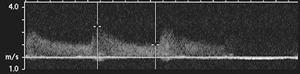
FIGURE 8-29 High grade stenosis (A) with increased peak velocity and loss of the triphasic pattern (B).
Studies comparing color-assisted duplex sonography with angiography demonstrate excellent results in the aortoiliac, femoral, and popliteal arteries. Results reported for sonography in the infrapopliteal vessels are more controversial [63,179,215]. Large series demonstrate that comparing sonography to angiography for occlusion and lesions with greater than 50% stenosis the sensitivities and specificities for the aortoiliac region were 86% and 97%, 80% and 96% respectively for the femoral and popliteal arteries, and 83% and 84% respectively for the arteries distal to the popliteal artery [122]. Postoperative evaluation of grafts (Fig. 8-30) and prior angioplasty can also be effectively evaluated with ultrasound [20].
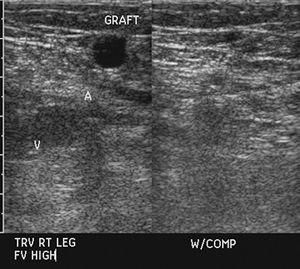
FIGURE 8-30 Ultrasound demonstrating normal flow in a vein graft in the femoral artery.
Ankle pressure measurements can be obtained when Doppler is used with blood pressure cuffs [6,128,151,190]. This allows for detection of low systolic pressures, useful information in predicting healing and in determining whether conservative therapy may be helpful [128,151]. Healing occurs in 92% of nondiabetic patients if ankle pressures are greater than 55 mm of mercury. Seventy-six percent of diabetic ulcers heal at this pressure level. Amputation is likely required when ankle pressures fall below this level [151]. Toe pressures are more useful in predicting healing in more distal ischemic disease. Doppler is accurate in most situations, but falsely elevated pressures can be seen in diabetic patients or in patients with other diseases that cause extensive vascular calcification [128,188,193]. Improved accuracy has been reported using Doppler techniques. Karanfilian et al. [111] compared laser Doppler velocimetry with conventional Doppler ankle pressures and transcutaneous oxygen measurements to determine which was most accurate in predicting healing of ischemic ulcers. Transcutaneous PO2 measurements were most accurate with a sensitivity of 100%, specificity of 88%, and accuracy of 95%. Laser Doppler studies were somewhat less reliable, but compared with Doppler ankle pressure the sensitivities were 79% and 75%, specificities were 96% and 26%, and accuracies were 87% and 52%, respectively [111].
Angiography
Color flow duplex combined with Doppler studies are useful and noninvasive, but, to date, angiography (conventional or digital) remains the gold standard for mapping vascular abnormalities (see Chapter 2) [73,196]. Angiography provides accurate information regarding the extent and sites of stenosis, pressure measurements or occlusion, the extent of involvement and collateral circulation, the anatomy of the vessel proximal and distal to the involved segment, prognostic information, and, when indicated, the appropriate level for amputation (see Figs. 8-21, 8-23, 8-24, and 8-31) [49,78,89,119,182,215].
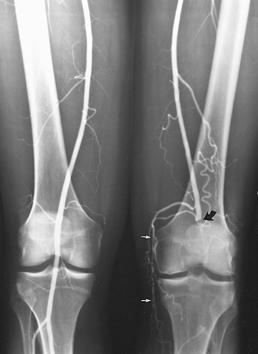
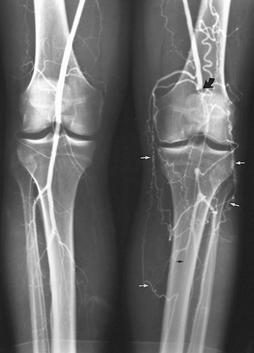
FIGURE 8-31 Angiogram images during run-off (A,B) demonstrating occlusion of the popliteal artery on the left (large curved arrow) with numerous collaterals (small white arrows). There is also an occlusion more distally (small black arrow) below the trifurcation.
Angiographic techniques also define vascular changes associated with specific disorders such as fibromuscular dysplasia and Buerger disease (see Figs. 8-23 and 8-24) [31,33,97,110,119].
Evaluation of the aorta, iliacs, and major vessels of the lower extremities can be easily accomplished using aortograms with run-off studies of the lower extremities or selective catheterization. However, smaller peripheral vessels in the feet and the pedal arch can be more difficult to identify, especially in the presence of small vessel disease [36,215]. The pedal arch, for example, is a low-resistance vascular bed with slow flow that can make angiographic filming difficult. Longer filming sequences or vasoactive drugs can be used to define these vessels to better advantage [11,169,199,216].
In recent years, the need for diagnostic angiography has been greatly reduced due to the improvements in CT and MR angiography [36,62,73,92,125,131,182]. In our department, digital subtraction angiography is most often performed when additional intervention may be required (thrombolysis, angioplasty, or stent placement).
Magnetic Resonance Angiography
Vascular imaging using MR techniques began using special pulse sequences with 2D time-of-flight or phase-contrast techniques [22]. These techniques were commonly degraded by artifacts (Fig. 8-32) including spin-saturation, turbulence, and step-ladder artifacts from reformatted thin-section images [22,88].
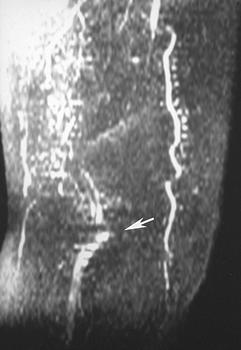
FIGURE 8-32 Coronal 2D time-of-flight (TOF) image just above the knees in a patient with popliteal artery occlusion. Multiple artifacts are due to slow flow and step-ladder artifacts in the popliteal region (arrow).
In recent years, multiple new approaches have resulted in improved evaluation of the smaller arteries in the foot and ankle [36,125,131,181,221]. Most approaches used today are performed with intravenous gadolinium, though intra-arterial gadolinium has also been studied [88,181,230]. Today, most MR imaging approaches use 3D bolus contrast injections (Fig. 8-33)[85,121]. Time of the contrast arrival to the area of interest is critical for optimal image quality [221]. Multiple methods have been described in the literature. Some are vendor related and others may be more universally used. Timing can be guessed (roughly 30 seconds from the antecubital fossa to the abdominal aorta) or more optimally, with a test dose to measure the peak contrast time. This is especially important for the small vessels of the foot and ankle [36]. Some MR systems can automatically track contrast arrival and initiate the imaging [221]. When this is not available, a test dose can be given. Several approaches have been described. Chomel et al. [36] imaged the malleolar region at 1-second intervals for 70 seconds following a small bolus test injection. The study was performed at the optimal contrast enhancement at that level. Tatli et al. [221] described a test injection consisting of 2 mL of contrast followed by 20 mL normal saline bolus using the same injection rate as planned for the examination. Images in the area of interest were performed every 2 seconds and analysis was performed to detect the time of peak enhancement.
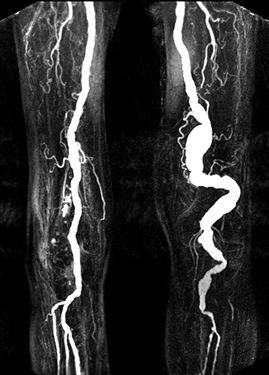
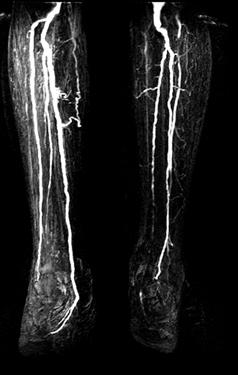
FIGURE 8-33 Popliteal artery aneurysm. MR angiogram images through the thigh (A) and calf (B) demonstrate the aneurysm on the left.
Imaging parameters vary with MR vendor. However, ultra-short TEs and TRs and high performance gradients are essential for high quality MR angiography [36,62,221]. Most studies are currently performed on optimized 1.5T units, but with time the needed technology changes on 3T units could offer improved signal-to-noise ratios [62]. Three-dimensional imaging can be performed with short TE/TR spoiled gradient echo sequences. Oblique coronal image planes are most useful for the leg or thigh as the largest field of view with the shortest scan time can be accomplished. The sagittal plane is more optimal for the foot [62]. The approach varies with the anatomic region. For evaluation of the entire lower extremity moving table approach which images at each station. Hybrid techniques incorporate both a single station bolus 3D angiogram and a second injection with two stations to evaluate the pelvis and thighs [221].
Regardless of the approach, 3D contrast-enhanced MR angiography techniques compare favorably to conventional angiography [36,62,131,181,182,221]. Sensitivities of 92% to 97% and specificities of 89% to 98% have been reported [92,152]. In addition, MR angiography has fewer adverse effects, especially in older or diabetic patients, compared to conventional angiography [126]. The American College of Radiology (ACR) appropriateness criteria rank MR angiography and conventional angiography equally (8) with CT angiography slightly lower (6) for patients with claudication [195]. The appropriateness criteria for posttreatment imaging consider MR angiography a useful screening procedure when additional intervention is not a consideration [83].
Computed Tomography Angiography
Multidetector CT (MDCT) angiography along with MR angiography has replaced conventional diagnostic angiography in many practices unless angiographic intervention may be required. The ACR appropriateness criteria ranks CT angiography (6) just below MR angiography (8) for peripheral vascular disease [83,195]. MR angiography may not be appropriate in patients with metallic implants or electrical devices. In addition, MR angiography cannot effectively evaluate vessel wall thickness or calcification [170]. CT angiography is an alternative in patients when MR imaging is contraindicated or assessment of mural disease is required [85,130,147]. Advantages of CT angiography compared to conventional angiography include that it is minimally invasive (intravenous contrast), acquires 3D data, can visualize calcified plaque and there is dramatically shorter examination time [5,73,148,192,198,234]. Reported scan times vary from 31.5 to 70 seconds [5,198].
CT angiography is an effective technique for evaluation of peripheral vascular disease, aneurysms, and traumatic disruption (Fig. 8-34) [5,73,148,234]. Hemodynamically significant stenoses (≥50%) are detected with sensitivities ranging from 93% to 99% and specificities of 95% to 99% with CT angiography [5,170]. Mural calcifications can be assessed with conventional axial CT images. However, using CT angiography, the presence of extensive calcifications decreases the accuracy [170]. Extensive vascular calcification reduces accuracy related to the beam hardening artifacts (Fig. 8-35) [170]. In this setting, MR angiography is the preferred technique.
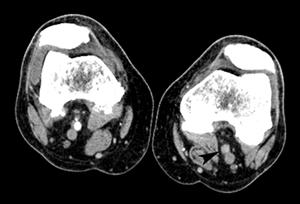
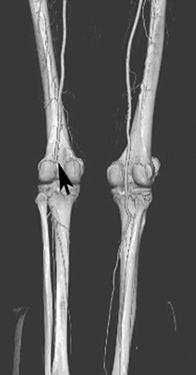
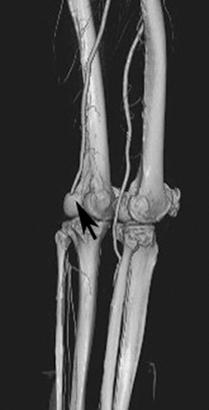
FIGURE 8-34 3D CT angiogram demonstrating embolic occlusion of the popliteal artery.A) Axial postcontrast CT image demonstrating no contrast in the left popliteal artery (arrowhead). Threedimensional CT angiogram images (B,C) demonstrate occlusion (arrow) with no collateral vessels.
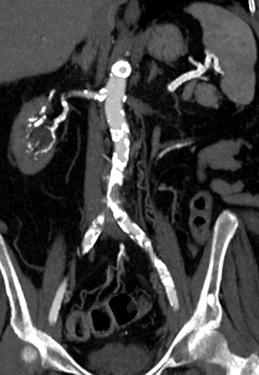
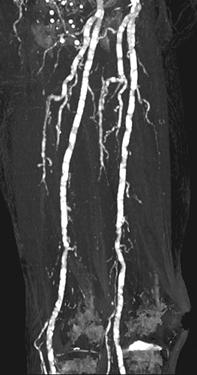
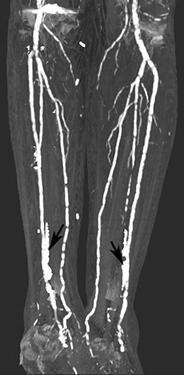
FIGURE 8-35 CT angiogram with extensive vascular calcification. CT angiogram images of the aortoiliac (A), femoral (B), and trifurcation vessels (C) demonstrating extensive mural calcifications with artifact (arrows) distally.
Similar to MR angiography, the timing of contrast and imaging in the region of interest is critical 32. Today, most scanners are equipped with bolus tracking software that optimizes vascular detail. Contrast volume may vary with the region of interest, but for the lower extremity 100 mL of nonionic contrast is injected and followed with a 70 mL normal saline flush. Protocols vary with equipment, but in most cases 120 kV and 140 mAs are used with rotation time of 0.5 seconds and table feed of 40 mm/sec. Reconstructions vary, but are typically 2 mm at 1.2 mm intervals [5,170,198].
Treatment
Treatment of soft tissue ischemic disorders depends on whether the process is primary or related to a systemic disease. Treatment also differs significantly between occlusive and vasospastic diseases.
Vasospastic Diseases
Patients with vasospastic conditions (see Table 8-5) are usually treated conservatively. For example, patients with Raynaud’s phenomenon should be instructed to protect their hands and feet properly in cold weather and to avoid smoking and vasoconstricting substances [65]. Patients with acrocyanosis and livedo reticularis should also be protected from cold exposure. Sympathetic blocking medications may be required in some patients [65]. Some physicians prefer to utilize calcium channel blockers such as nifedipine. Other medications include angiotensin II inhibitors and nitrites [180]. Most patients (90% of patients with Raynaud’s) respond and improve with these conservative measures [107,180].
In some patients, conservative measures are not successful. In this setting, more aggressive therapy may be indicated. Although the role of sympathectomy is unclear, this procedure may be useful in selected cases. Janoff et al. [107] noted a successful response to sympathectomy in 10 patients with refractory Raynaud’s phenomenon and painful lower extremity vasospasm.
Vascular Occlusive Diseases: Arterial
Treatment of vascular stenosis and occlusion depends on the cause, the location (proximal or large vessel vs. distal small or microvascular disease), and the patient’s medical status. Therapy generally falls into three categories: conservative, percutaneous intervention, or surgery [8,13,17,19,38,51,84,105,115,161,164,194,207,217,218].
Conservative therapy is used when possible, especially in patients with small vessel disease, such as, in diabetic patients, or in patients with peripheral ulcers and minor infections. These patients should have ulcer debridement, be given appropriate footwear to reduce pressure on ulcerated areas, and be given appropriate antiseptic or antibiotic therapy [17,165,173,188]. In the absence of significant vascular disease, larger soft tissue ulcerations can be treated with skin grafts, local flaps, or vascularized muscle flaps [17].
Two therapeutic percutaneous techniques have been commonly employed over the years. Percutaneous transluminal angioplasty (PTA) and thrombolysis, either mechanical or chemical, have both proven useful and provide options to bypass surgery [8,51]. More recently, additional procedures include angioplasty with stent placement, cutting-balloon angioplasty, cryoplasty, and laser atherectomy [8]. Treatment decisions are related to patient status and disease location. Decision making is based on location and extent of disease. Typically, these decisions are based on whether the disease involves the aortoiliac, femoropopliteal, or infrapopliteal vessels [8].
Endovascular therapy is most effective for iliac disease (Fig. 8-36). Current percutaneous angioplasty techniques are successful in 88% of patients with a 5-year posttreatment patency of 66%. Success rates are even higher with combined stent placement (39% reduction in long-term failures) [8]. Endotherapy for iliac disease is less successful for long lesions (≥5 cm), when iliac aneurysms are present and in the presence of severe bilateral aortoiliac disease [8,19,164].
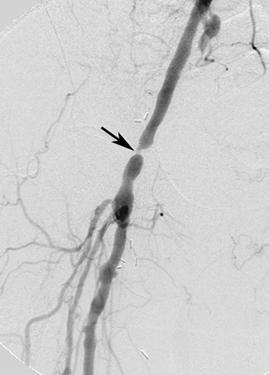
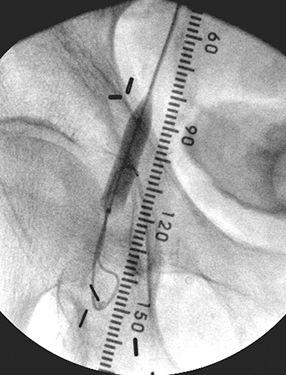
FIGURE 8-36 Iliac angioplasty.A) Digital subtraction angiogram demonstrates a short segment high grade stenosis (arrow) in the iliac artery.B) Image taken during balloon dilatation.
Stenosis is more common in the iliac artery while more extensive long segment disease is more prevalent in the femoropopliteal segments. These segments have higher resistance, reduced flow rates, and are more prone to spasm [8]. The failure rate of endovascular procedures is higher [8,51].
Whereas imaging of the proximal leg vasculature is straightforward, meticulously performed angiography of the tibial arteries and, particularly, the arteries of the foot and ankle is essential in delineating appropriate target sites for distal intervention [8,17,125]. This may require selective antegrade catheterization, delayed image acquisition, use of intra-arterial vasodilators, and digital subtraction angiography with magnification [8,109,116]. In addition, the identification of adequate arterial outflow distal to the site of intervention is a major determinant of success for both distal surgical bypass and endoluminal procedures [8,15,30,49]. This is particularly true for the inframalleolar interventions in which pedal outflow is mandatory for success [12].
Though not commonly performed in our practice, development of microcatheters with mounted balloons less than 4 mm in diameter has permitted angioplasty not only of the tibial and peroneal arteries proper, but also of the pedal branches of these vessels (Fig. 8-37). Because of the diffuse nature of atherosclerotic disease of the lower extremities, patient selection based on the anatomy depicted on preliminary arteriography is the critical step in ensuring a successful outcome for tibial and peroneal angioplasty. Most patients with critical lower leg ischemia do not have a disease distribution appropriate for percutaneous management and are better served with operative intervention [8,17]. Anatomic features predicting a successful outcome to infrapopliteal percutaneous angioplasty include the presence of fewer than four to five stenoses or an occlusion less than 6 cm long in a single tibial or peroneal artery. In addition, there should not be significant proximal vessel disease [227].
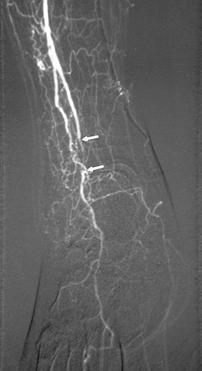
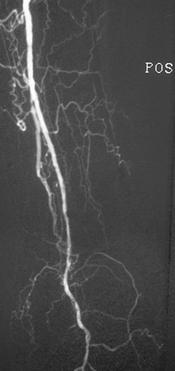
FIGURE 8-37 Angiograms before (A) and after (B) angioplasty of a short occlusion (arrows in A) of the anterior tibial artery.
Using careful technique in patients with relatively focal infrapopliteal disease and potentially restorable run-off below the site of angioplasty, endoluminal therapy should result in a technical success rate of greater than 90% [98,199,223]. A limb salvage rate of approximately 80% should be expected [15,223]. Successful limb salvage is optimized if straight-line flow to the foot is established, the patients are nondiabetic, and gangrene is absent at the time of the procedure [15,199,223].
Complications associated with percutaneous angioplasty are infrequent, but primarily they include thrombosis and distal embolization. These complications may be readily managed percutaneously using thrombolysis or suction embolectomy. The possibility for procedural complications can be reduced by the periprocedural administration of antiplatelet agents, heparin, and vasodilators [8,9,51]. Fortunately, a failed percutaneous angioplasty or the occurrence of a procedural complication rarely precludes the performance of bypass graft surgery or alters previously planned surgery.
Thrombolysis.
The discovery of effective thrombolytic agents and the development of sophisticated catheter delivery systems have encouraged increased utilization of nonoperative techniques for treating peripheral vascular occlusive disease [8,39,54,75,79,80,95,99,113,127,171,222]. Thrombolytic therapy plays an important role not only as a primary treatment for these vascular occlusions, but also as an adjunct to percutaneous angioplasty when the patient has an underlying focal stenosis. The basis of thrombolysis lies in the ability of exogenously administered agents to dissolve unwanted clot. The action of these agents is targeted toward the lysis of clot fibrin. Fibrinolysis is mediated by the proteolytic enzyme plasmin, which is effective in breaking down fibrin into its inactive degradation products. Plasmin is derived from activation of an inactive proenzyme called plasminogen, which is normally circulating with plasma. Thrombolytic therapy has evolved over the years with new agents and techniques. Agents capable of activating plasminogen include streptokinase (SK), urokinase (UK), tissue plasminogen activator (t-PA), recombinant tissue plasmin activator (rt-PA), third-generation plasminogen activator reteplase and glycoproteins IIb and IIIa platelet receptor antagonist abciximab [56,152,155,198,200,207,225]. Recent reports with third-generation agents demonstrate complete clot resolution in up to 92% of patients [56]. The patients included in the study were treated for thombosis of native arteries (36%) and graft thrombosis (64%). Ten percent of occlusions were embolic and 90% thrombotic [56]. Patients can be followed with Doppler ultrasound to evaluate treatment. Thirty days following treatment, Drescher et al. [56] reported 92% success rate with consistent clinical improvement.
Long-term patency following thrombolysis of lower extremity occlusions can be more variable. The longevity of treated proximal occlusions tends to exceed that of more distal occlusions, and grafts exhibit a more durable response than native vessels [153]. Vein grafts typically remain patent longer than synthetic grafts [56]. Most important seems to be correction of the underlying lesion responsible for vessel thrombosis. Long-term patency for lysed vessels with a corrected lesion far exceeds patency of those in which the stenotic lesion remains uncorrected. In the case of grafts, older grafts appear to have greater long-term patency than newer grafts following thrombolysis [8,56].
Patient selection is critical in order to avoid hemorrhagic complications. The incidence of this complication is reported to range from 1% for hemorrhagic stroke to 5% for major hemorrhages. Minor hemorrhage occurs in up to 15% of patients [8,207]. Drescher et al. [56] reported major hematomas in 12% of patients that required transfusions and 8% with hematomas at the puncture site. Hematuria occurred in 4% of patients and 4% of patients developed thrombocytopenia due to decreased platelet count. An additional 8% of patients developed distal emboli following treatment of the thrombosis. Two percent of patients developed a stroke just over a month following treatment [56].
As noted above, distal embolization of the thrombus during lysis of a lower extremity clot is one of the most uniformly reported complications and occurs in 5% to 50% of cases [112,225]. Identification, in most cases, requires careful angiographic evaluation, especially of the ankle and foot vessels. This unavoidable complication fortunately responds well to continuation of thrombolytic infusion [112,225]. Although emboli may be asymptomatic, dramatic worsening of lower extremity ischemia during treatment should indicate that embolization has occurred. Fortunately, irreversible tissue necrosis and occlusion of distal run-off preventing subsequent surgical reconstruction occur only rarely [225].
Surgical Intervention
The goal of any infrapopliteal arterial intervention, regardless of whether it is percutaneous angioplasty or surgery, is the permanent relief of ischemic symptoms. Surgical interventions for peripheral vascular disease primarily involve bypass graft techniques and endarterectomy [8,48,69,71,108,224,227,235]. Bypass grafts are most commonly employed in distal or diffuse occlusions while endarterectomy may be preferred for aortoiliac or proximal femoral disease [8]. Vascular stents have been successfully used in the aorta and iliac vessels. Autogenous vein grafts are more commonly used in the infrainguinal regions (Fig. 8-38). Vein grafts are reversed so the valves to not obstruct flow. Vein grafts demonstrate patency rates of 70% to 80% over 5 years which is more favorable than prosthetic grafts [4].
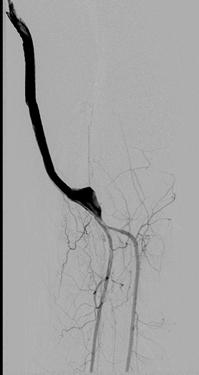
FIGURE 8-38 Digital subtraction angiogram demonstrating a vein bypass graft to the trifurcation vessels.
Atheroembolus to the lower extremity arterial system is a unique type of occlusive disease in which the source of the embolus, rather than the embolus itself, is of primary therapeutic concern [178]. Aortic aneurysms and occlusive aortoiliac disease are the most common sources of atheroemboli [114,117,125]. Plaques in these regions are prone to degeneration with showering of cholesterol, calcium, and thromboembolic debris to the distal circulation. The shaggy aorta, which is lined with irregular, ulcerated plaques, seems to be particularly strongly implicated in atheroembolication [114]. These emboli have a propensity to travel to the small arteries of the foot and ankle, where they may give rise to the classic blue toe syndrome [28]. If allowed to proceed unabated, atheroembolization can result in limb- threatening ischemia. There is no role for percutaneous, nonsurgical interventions in atheroembolic disease. Surgical correction consists of exclusion by bypass or removal of the source by endarterectomy [8,114].
Treatment of arterial insufficiency in the foot and ankle may be a complex undertaking, especially when atherosclerosis is involved. Because atherosclerosis tends to be a diffuse disease, there is the potential for multiple sequential sites of vascular stenosis proximal to the foot and ankle that need to be addressed to optimize therapeutic outcome (see Fig. 8-38) [227]. Treatment of traumatic lower extremity arterial lesions must be individualized, depending on the physical examination and mode of injury [70,73].
Postoperative evaluation of patients with prior endarterectomy or bypass grafts can be accomplished with ultrasound, MR and CT angiography, and conventional angiography. The latter is most useful if additional intervention may be indicated [8,192,221,232,233]. Detection of graft patency issues is important before thrombosis occurs. For the most part, noninvasive modalities such as the ABI and arterial duplex ultrasound are utilized. Ultrasound is more useful in most situations because it is can enable one not only to diagnose impending intervention failure, but also to identify the actual site responsible for the failure [20,212,213,226].
Ultrasound is capable of detecting those grafts with a high likelihood of failure. A peak systolic velocity measurement less than 40 or 45 cm/sec within the graft strongly suggests impending graft failure, but it is not specific as to cause [16,18,101]. By using color-flow Doppler sonography, the entire length of the bypass graft may be examined in real time, and sites of suspected stenosis may be identified and quantified using Doppler spectral analysis. The severity of stenoses is graded according to a peak systolic ratio. This is calculated by dividing the peak systolic velocity at the site of suspected stenosis by that measured in the normal graft 2 to 4 cm more proximally. Velocity ratios of 2 or greater correspond to a 50% diameter reduction, whereas ratios of 3 or greater indicate a 75% stenosis [20,135,178]. Peak systolic velocity ratios greater than 3 generally indicate impending bypass occlusion and warrant intervention [135,178].
Graft failure occurs for a variety of reasons, depending on the interval that has passed since the operation. Early failures are apparent within 1 month of surgery and are usually ascribed to technical errors. These include suture placement errors, graft kinking, clamp injuries, residual venous side branches, poorly lysed venous graft valves, and poor selection of the anastomotic sites. Intermediate failures occur during the first 1 to 2 years and are attributed to the formation of fibrointimal hyperplasia at the anastomotic sites or within the graft conduit, usually at a venous valve site. Late failures, beyond 2 years, are believed to be a result of the continued atherosclerotic disease process in the native vessels proximal and distal to the anastomosis. The purpose of graft surveillance is to detect intragraft or perianastomotic abnormalities before graft thrombosis can occur [16,135].
Failed or failing revascularization interventions can be managed in several ways. The method selected depends on the type of revascularization performed, the length of time since intervention, and the cause of the failure [21,55,74,153]. Surgical grafts to the lower leg are usually of autologous vein. Early graft failures (less than 30 days postoperatively) usually present with graft thrombosis that is due to a technical problem associated with graft placement. These patients typically return directly to the operating room for a directed graft revision and removal of thrombus. If the precipitating cause of failure is poor outflow, an additional or replacement graft may be required. Percutaneous angioplasty is not indicated in these patients [94].
Vein graft problems occurring in the intermediate postoperative period (1 to 2 years) are most common and are usually the result of an intrinsic graft lesion, such as fibrointimal hyperplasia at the anastomotic sites or at a venous valve. Stenotic lesions occur in 20% to 30% of grafts [21,164]. Various surgical interventions are available and include patch angioplasty, jump graft extension, and excision with either primary reanastomosis or interposition vein graft [8,18]. Excision of the abnormal vein segment with primary anastomosis or interposition grafting restores hemodynamics to a more natural state and may provide the more durable secondary patency [16].
If a failing graft is identified before thrombosis occurs, a surgical secondary patency rate of approximately 85% can be expected [16]. Unfortunately, once graft thrombosis has occurred, surgical secondary patency rates fall precipitously, with 1-year patency rates reported as low as 40% [16,74]. Replacement of the clotted vein graft (secondary revascularization) has been advocated by many, with 5-year patency rates of 55% to 65% being achieved when suitable autologous vein is available. Results using synthetic replacement grafts are not as satisfactory [16,74,164].
Grafts that fail in the late postoperative period (more than 2 years) may do so because of intrinsic graft lesions, although this is not common. Usually, late failure is due to the progression of atherosclerosis in the native arterial inflow to the graft, at the anastomotic sites, or in the arterial run-off distal to the graft. Arteriographically proven anastomotic narrowings usually benefit from surgical revision, whereas occlusive disease proximal or distal to the graft may require some sort of secondary grafting procedure or percutaneous angioplasty [16,74,164]. Percutaneous transluminal interventions (Fig. 8-39) may possess some role in the management of patients with jeopardized lower leg bypass grafts. Because early graft failures are usually technical in nature, there is really no indication for percutaneous therapy in that patient group. The role of percutaneous therapy for grafts failing in the intermediate postoperative period has been examined, with controversial results. Several investigators have had good immediate technical success and long-term patency of graft stenoses treated with percutaneous angioplasty [79,212]. Midgraft stenosis at valve sites, as opposed to anastomotic stenoses, appear to require higher balloon inflation pressure, longer dilatation times, and over dilatation to achieve success [212].
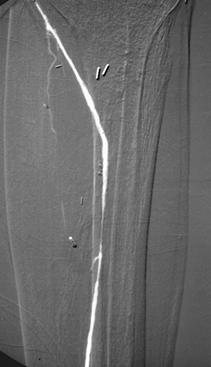
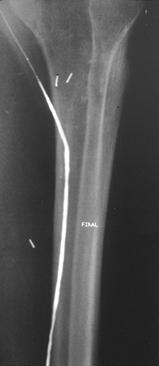
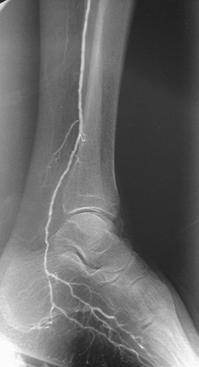
FIGURE 8-39 Angiograms before (A) and after (B) angioplasty for stenosis just distal to a femoroposterior tibial artery vein bypass graft. Lateral images (C) of the ankle and hindfoot show the improved run-off distally.
The majority opinion is that percutaneous angioplasty of bypass graft stenosis achieves a high initial technical success rate, but the results are not durable because fibrointimal hyperplasia, unlike atherosclerotic narrowing, exhibits a tendency for elastic rebound following dilatation [16,173,175,203–205]. Disparate results from these PTA studies may be a reflection of the composition of material causing the graft stenosis. With anastomotic stenoses resulting from progressive atherosclerosis, rather than fibrointimal hyperplasia, angioplasty results may be better than if the stenosis is composed entirely of hyperplastic tissue. Varying combinations of fibrointimal hyperplasia and atherosclerosis have been retrieved from these stenotic sites [52,55]. Percutaneous transluminal directional atherectomy devices have been used to remove the noncompressible material causing graft anastomotic stenoses. The experience is limited, but this technique has generated results superior to those for percutaneous angioplasty alone and approaching those reported for surgical revision [52,55]. Adjunctive percutaneous angioplasty following atherectomy is frequently necessary to achieve optimal results [52,55].
Perhaps the most important contribution percutaneous angioplasty can offer is in patients with grafts that have failed or are failing in the late postoperative period. Because late graft failures are frequently secondary to progressive narrowing of graft in-flow or run-off vessels, PTA (see Fig. 8-39) can be used to extend the life of lower extremity bypass grafts [16]. In addition, percutaneous endoluminal procedures may benefit patients who have no suitable veins available for graft revision or replacement. If percutaneous interventions are utilized in intermediate or late graft problems, postprocedure surveillance with ultrasound is critical for continued graft maintenance [18].
More aggressive surgical approaches are often required in patients where revascularization or endoluminal procedures fail. Diabetic patients are the best example [17]. Diabetic patients account for the majority of ischemic complications in the foot and ankle. From 55% to 75% of diabetics require vascular reconstruction [17]. Nonhealing skin wounds and ulcerations may require repair with vascularized muscle flaps [17,219]. More than 80% of major amputations occur in the diabetic population [17].
REFERENCES
- Abramson KI, Lerner D, Shumacker HB, et al. Clinical picture and treatment of later stage trenchfoot. Am Heart J. 1946;32:52–71.
- Adar R, Papa MZ, Halpern Z, et al. Cellular sensitivity to collagen in thromboangiitis obliterans. N Engl J Med. 1983; 308:1113–1121.
- Adeyokunnu AA. Bilateral gangrene of the feet associated with salmonella infection in children with sickle cell anemia. Ann Trop Pediat. 1982;2:41–43.
- Albers M, Romiti M, Brochado-Neto FC, et al. Metaanalysis of alternate autologous vein bypass grafts to infrapopliteal arteries. J Vasc Surg. 2005;42:449–455.
- Albrecht T, Foert E, Holtkamp R, et al. 16-MDCT angiography of aortoiliac and lower extremity arteries: comparison with digita subtraction angiography. Am J Roentgenol. 2007; 189:702–711.
- Allen J, Oates CP, Henderson J, et al. Comparison of lower limb arterial assessments using color-duplex ultrasound and ankle/brachial index measures. Angiology. 1996;47: 225–232.
- Almahameed A, Pinto DS. Pernio (chilblains). Curr Treat Options Cardiovasc Med. 2008;10:128–135.
- Almahameed A, Bhatt DL. Contemporary management of peripheral arterial disease: III. Endovascular and surgical management. Cleveland Clin J Med. 2006;73:S45–S51.
- Altin RS, Flicker S, Naidech HJ. Pseudoaneurysm and arteriovenous fistula after femoral catheterization. Association with low femoral punctures. Am J Roentgenol. 1989;152: 629–631.
- Anderson ME, Allen PD, Moore T, et al. Computerized nailfold video capillaroscopy—a new tool for assessment of Raynaud’s phenomenon. J Rheumatol. 2005;32:841–848.
- Andros G, Harris RW, Dulawa LB. Need for arteriography in diabetics with gangrene and palpable foot pulses. Arch Surg. 1985;119:1260–1263.
- Andros G, Harris RW, Salles-Cunha SX, et al. Bypass grafts to the ankle and foot. J Vasc Surg. 1988;7(6):785–794.
- Ascer E, Veith FJ, Gupta SK. Bypasses to plantar arteries and other tibial branches. An extended approach to limb salvage. J Vasc Surg. 1988;8:434–441.
- Babb RR, Alacon-Segovia D, Fairbai JF. Erythromelalgia. Review of 51 cases. Circulation. 1964;29:136–142.
- Bakal CW, Sprayregen S, Scheinbaum K, et al. Percutaneous transluminal angioplasty of the infrapopliteal arteries. Results in 53 patients. Am J Roentgenol. 1990;154:171–174.
- Bandyk DF, Bergamini TM, Towne JB, et al. Durability of vein graft revision. The outcome of secondary procedures. J Vasc Surg. 1991;13:200–210.
- Bandis JC, Derr JW, Richardson JD. A rational approach to ischemic and ischemic-diabetic foot reconstruction. Op Tech Plastic Reconst Surg. 1997;4:217–235.
- Bandyk DF, Jorgensen RA, Towne JB. Intraoperative assessment of in situ saphenous vein arterial bypass grafts using pulsed Doppler spectral analysis. Arch Surg. 1986;121:292–299.
- Beard JD. ABC of arterial and venous disease: chronic lower limb ischemia. Br Med J. 2000;320:854–857.
- Begelman SM, Jaff MR. Noninvasive diagnostic strategies for peripheral arterial disease. Cleveland Clin J Med. 2006; 73(suppl):S22–S29.
- Belkin M, Donaldson MC, Whittemore AD, et al. Observations on the use of thrombolytic agents for thrombotic occlusion of infra-inguinal vein grafts. J Vasc Surg. 1990;11: 289–296.
- Berquist TH. MRI of the Musculoskeletal System. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Berquist TH. Diagnostic and therapeutic injections as an aid to musculoskeletal diagnosis. Semin Intervent Radiol. 1993;10:326–343.
- Berquist TH. Imaging of Orthopedic Trauma. 2nd ed. New York, NY: Raven Press; 1992.
- Blair H. Comminuted fractures and fracture dislocation of the body of the astralagus. Am J Surg. 1943;59:3743.
- Bluemke DA, Zerhouni EA. MRI of avascular necrosis of bone. Topics Magn Reson Imaging. 1996;8:231–246.
- Boyko EJ, Ahroni JH, Davignon D, et al. Diagnostic utility of the history and physical examination for peripheral vascular disease among patients with diabetes mellitus. J Clin Epidemiol. 1997;50:659–668.
- Brewer ML, Kinnison ML, Perler BA, et al. Blue toe syndrome. Treatment with anticoagulants and delayed percutaneous transluminal angioplasty. Radiol. 1988;166: 31–36.
- Brower AC. The osteochondrosis. Orthop Clin North Am. 1983;14:99–117.
- Brown KT, Schoenberg NY, Moore ED, et al. Percutaneous transluminal angioplasty of infrapopliteal vessels. Preliminary results and technical considerations. Radiology. 1988;169:75–78.
- Bull PG, Mendel H, Hold M, et al. Distal popliteal and tibioperoneal transluminal angioplasty. Long-term follow-up. J Vasc Interv Radiol. 1992;3:45–53.
- Bush WH, Swanson DP. Acute reactions to intravascular contrast media: types, risk factors, recognition, and specific treatment. Am J Roentgenol. 1991;157:1153–1161.
- Campbell WB, Fletcher EL, Hands LJ. Assessment of distal lower limb arteries. A comparison of arteriography and Doppler ultrasound. Ann Royal Coll Surg. 1986;68:37–39.
- Canale ST, Kelly, FB. Fractures of the neck of the talus. J Bone Joint Surg. 1978;60A:143–156.
- Chiodo CP, Herbst SA. Osteonecrosis of the talus. Foot Ankle Clin N Am. 2004;9:745–755.
- Chomel S, douek P, Moulin P, et al. Contrast-enhanced MR angiography of the foot: anatomy and clinical application in patients with diabetes. Am J Roentgenol. 2004;182:1435–1442.
- Chryssanthow CP. Dysbaric osteonecrosis. Etiologic and pathologic concepts. Clin Orthop. 1978;130:94–106.
- Cisek PL, Eze AR, Comerota AJ, et al. Microcirculatory compensation to progressive atherosclerotic disease. Ann Vasc Surg. 1997;11:49–53.
- Cleveland TJ, Cumberland DC, Gaires PA. Percutaneous aspiration thromboembolectomy to manage the embolic complications of angioplasty and as an adjunct to thrombolysis. Clin Radiol. 1994;49:549–552.
- Comerford J, Dawsett G, Kennedy J, et al. 111-Indium labeled platelet scanning in diagnosis of microembolic disease. A prospective study. Irish J Med Sci. 1986;155:121–122.
- Coni NK. Posture and arterial pressure in the ischemic foot. Age Aging. 1983;12:151–154.
- Coughlin MJ, Mann RA, Saltzman CL. Surgery of the Foot and Ankle. 8th ed. St. Louis, MO: Mosby/Elsevier; 2007.
- Cragg AH, Smith TP, Corson JD, et al. Two urokinase dose regimens in native arterial and graft occlusions. Initial results of a prospective, randomized clinical trial. Radiology. 1991;178:681–686.
- Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386.
- Cronberg CN, Sjoberg S, Albrechtsson U, et al. Peripheral arterial disease: contrast-enhanced 3-D MR angiography of the lower leg and foot compared with conventional angiography. Acta Radiologica. 2003;44:59–66.
- Dahlin DC, Ivins JC. Fibrosarcoma of bone. A study of 114 cases. Cancer. 1969;23:35–45.
- Dalinka MK, Edeiken J, Finkelstein JB. Complications of radiation therapy in adult bone. Semin Roentgenol. 1974;9: 29–39.
- Dardik H, Ibrahim IM, Sussman B, et al. Morphologic structure of the pedal arch and its relationship to patency of crural vascular reconstruction. Surg Gynecol Obstet. 1981;152:645–648.
- Darvin HI, King TA. Lower extremity arterial occlusive disease. Clin Podiatr Med Surg. 1992;9:69–77.
- Davidson JK. Aseptic Necrosis of Bone. Amsterdam: Excerpta Medica; 1976.
- Deutschmann Ha Schoellnast H, Temmel W, et al. Endoluminal therapy in patients with peripheral vascular disease: prospective assessment of quality of life in 190 patients. Am J Roentgenol. 2007;188:169–175.
- deWolf VG. Assessment of circulation in occlusive arterial disease of the lower extremities. Mod Concepts Cardiovasc Dis. 1976;45:91–95.
- Di Giovanni CW, Patel A, Calfee R, et al. Osteonecrosis in the foot. J Am Acad Orthop Surg. 2007;15:208–217.
- Dobrin PB. Mechanisms and prevention of arterial injuries caused by balloon embolectomy. Surgery. 1989;106:457–466.
- Dolmatch BL, Gray RJ, Horton KM, et al. Treatment of anastomotic bypass graft stenosis with directional atherectomy. Short-term and intermediate term results. J Vasc Interv Radiol. 1995;6:105–113.
- Drescher P, McGuckin J, Rilling WS, et al. Catheter-directed thrombolytic therapy in peripheral artery occlusions: combining retephase and abciximab. Am J Roentgenol. 2003;180:1385–1391.
- Durant JH, Edwards WS. Small vessel occlusion in the extremity after various periods of arterial obstruction. An experimental study. Surgery. 1973;73–240.
- Eichhorn J, Sima D, Lindshhau C, et al. Antiendothelial cell antibodies in thromboangiitis obliterans. Am J Med Sci. 1998;315:17–23.
- Edmonds ME. The diabetic foot. Pathophysiology and treatment. Clin Endocrinol Metab. 1986;14:889–916.
- Edwards EA, Cooley MH. Peripheral vascular symptoms as an initial manifestation of polycythemia vera. JAMA. 1970; 214:1463–1467.
- Elliot DH, Harrison JAB Bone necrosis occupational hazard of diving. J R Nav Med Serv. 1970;56:140–161.
- Ersoy H, Rybicki FJ. MR angiography of the lower extremities. Am J Roentgenol. 2008;190:1675–1684.
- Favaretto E, Pili C, Amato A, et al. Analysis of agreement between duplex ultrasound scanning and arteriography in patients with lower limb artery disease. J Cardiovasc Med. 2007;8:337–341.
- Feldaker M, Hines EA, Kierland RR. Livedo reticularis with ulcerations. Circulation. 1956;13:196–216.
- Feller SR, Docherty GL. Vasospastic disease. Diagnosis and management. Clin Podiatr Med Surg. 1986;3:463–470.
- Ficat RF, Arlet J. Ischemia and Necrosis of Bone. Baltimore, MD: Williams & Wilkins; 1980.
- Fisher DF, Bichek WH, Holley KE, et al. Corticosteroid induced aseptic necrosis. Experimental study. Clin Orthop. 1972;84:200–206.
- Freiberg AH. Infarction of the second metatarsal bone. A typical injury. Surg Obstet Gynecol. 1914;19:191–193.
- Frykberg ER, Crump JM, Dennis JW, et al. Nonoperative observation of clinically occult arterial injuries. A prospective evaluation. Surgery. 1991;109:85–96.
- Frykberg ER, Crump JM, Vines FS, et al. A reassessment of the role of arteriography in penetrating proximal extremity trauma. A prospective study. Trauma. 1989;29: 1041–1052.
- Furcy JG, Ferrell-Torells M, Regan JW. Fibrosarcoma arising at the site of bone infarcts. J Bone Joint Surg. 1960;42A: 802–810.
- Gabriel A, Camp MC, Paletta C, et al. Vascular ulcers. E-medicine, updated August 19, 2008. Available at: http://emedicine.emedscape.com.
- Gakhal MS, Sartip KA. CT angiography signs of lower extremity vascular trauma. Am J Roentgenol. 2009;193: W49–W57.
- Gardiner GA, Koltun W, Kandarpa K, et al. Thrombolysis of occluded femoropopliteal grafts. Am J Roentgenol. 1986;147: 621–626.
- Gardiner GA Jr, Sullivan KL. Complications of regional thrombolytic therapy. In: Kadir S, ed. Current Practice of Interventional Radiology. Philadelphia, PA: BC Decker; 1991:87–91.
- Gasper MR. Arterial embolism and thrombosis. Major Probl Clin Surg. 1981;4:158–175.
- Gayraud M. Raynaud’s phenomenon. Joint Bone Spine. 2007;74:e1–e8.
- Gloviczki P, Morris SM, Bower TC, et al. Microvascular pedal bypass for salvage of the severely ischemic limb. Mayo Clin Proc. 1991;66:243–253.
- Graor RA, Risius B, Lucas FV, et al. Thrombolysis with recombinant human tissue-type plasminogen activator in patients with peripheral artery and bypass graft occlusions. Circulation. 1986;74(suppl):15–20.
- Graor R, Risius B, Young JR, et al. Low dose streptokinase for selective thrombolysis. Systemic effects and complications. Radiology. 1984;152:35–39.
- Gregg PJ, Walder DN. Scintigraphy vs. radiography in early diagnosis of bone necrosis. J Bone J Surg. 1980;62B: 214–221.
- Greyson ND, Kassel EE. Serial bone scan changes in recurrent bone infarction. J Nucl Med. 1976;17:184–186.
- Grollman JH, Bettmann MA, Boxt LM, et al. ACR appropriateness criteria recurrent symptoms following lower extremity angioplasty. Revised 2005, available at: http://www.acr.org/ac.
- Gruntzig A, Kumpe DA. Technique of percutaneous transluminal angioplasty with the Gruntzig balloon catheter. Am J Roentgenol. 1979;132:547–552.
- Hai Z, Guangrui S, Yuan Z, et al. CT angiography and MRI in patients with popliteal artery entrapment syndrome. Am J Roentgenol. 2008;191:1760–1766.
- Hallett JW, Greenwood LH, Robinson JG. Lower extremity arterial disease in young adults. Ann Surg. 1985;202:647–652.
- Hallett JW, Yrizarry JM, Greenwood LH. Regional low dose thromboembolic therapy for peripheral arterial occlusions. Surg Obstet Gynecol. 1983;156:148–154.
- Hany TF, Schmidt M, Davis CP, et al. Diagnostic impact of four processing techniques in evaluating contrast-enhanced, three-dimensional MR angiography. Am J Roentgenol. 1998;170:907–912.
- Hausman M. Microvascular application in limb sparing tumor surgery. Orthop Clin N Am. 1989;20:427–437.
- Hawkins LG. Fractures of the neck of the talus. J Bone Joint Surg. 1970;52A:991–1002.
- Hawks SE, Pentecost MJ. Angiography and transcatheter treatment of extremity trauma. Semin Intervent Radiol. 1992;9:19–27.
- Herborn CU, Goyen M, Quick HH, et al. Whole-body 3D MR angiography of patients with peripheral arterial occlusive disease. Am J Roentgenol. 2004;182:1427–1434.
- Herrick AL. Pathogenesis of Raynaud’s phenomenon. Rheumatology. 2005;44:587–596.
- Hewes RC, White RI, Jr. Murray RR, et al. Longterm results of superficial femoral angioplasty. Am J Roentgenol. 1986; 146:1025–1029.
- Hicks ME, Picus D, Darcy MD, et al. Multilevel infusion catheter for use with thrombolytic agents. J Vasc Interv Radiol. 1991;2:73–75.
- Hight DW, Telneyn-Cough NP. Changing clinical trends in patients with extremity arterial disease. Surgery. 1976;79: 172–176.
- Hoffer EK, Sclafani SJ, Herskowitz MM, et al. Natural history of arterial injuries diagnosed with arteriography. J Vasc Interv Radiol. 1997;8:43–53.
- Horvath W, Oertl M, Haidinger D. Percutaneous transluminal angioplasty of crural arteries. Radiol. 1990;177:565–569.
- Huettl EA, Soulen MC. Thrombolysis of lower extremity embolic occlusions. A study of the results of the STAR registry. Radiol. 1995;197:141–145.
- Hungerford DS. Early diagnosis of ischemic necrosis of the femoral head. Johns Hopkins Med J. 1975;137:270–275.
- Idu MM, Blankenstein JD, de Gier P, et al. Impact of color flow duplex surveillance program on infra-inguinal vein graft patency. A five year experience. J Vasc Surg. 1993; 17:42–53.
- Ilfeld W, Rosen V. Osteochondritis of the first metatarsal sesamoid. A report of 3 cases. Clin Orthop. 1972;85:3841.
- Imhof H, Breitenseher M, Trattnig S, et al. Imaging of avascular necrosis of bone. Eur Radiol. 1997;7:180–186.
- Itin P, Stalder H, Vischer W. Symmetrical peripheral gangrene in disseminated tuberculosis. Dermatologica. 1986;173:189–195.
- Jackson JE, Mitchell A. Advanced vascular interventional techniques in the management of trauma. Semin Intervent Radiol. 1997;14:139–149.
- Jahss MH. The sesamoids of the hallux. Clin Orthop. 1981;157:88–97.
- Janoff KA, Phinney ES, Porter JM. Lumbar sympathectomies for lower extremity vasospasm. Am J Surg. 1985;150:147–152.
- Jeans WD, Armstrong S, Cole SE, et al. Fate of patients undergoing transluminal angioplasty for lower limb ischemia. Radiol. 1990;177:559–564.
- Karacagil S, Almgren B, Bergquist D. Patterns of atherosclerotic occlusive disease of lower leg and pedal arteries in hypertensive patients undergoing infra-inguinal bypass procedures. Int Angiol. 1996;15:57–60.
- Karacagil S, Almgren B, Bowald S, et al. A new method of angiographic runoff evaluation in femoro-distal reconstructions. Arch Surg. 1990;125:1055–1058.
- Karanfilian RG, Lynch TG, Ziral JT, et al. The value of laser Doppler velocimetry and transcutaneous oxygen determination in predicting healing of ischemic foot ulceration and amputation in diabetic and nondiabetic patients. J Vasc Surg. 1986;4:511–516.
- Katzen BT. Technique and results of low-dose infusion. Cardiovasc Intervent Radiol. 1988;11:S41–S47.
- Kaufman JA, Bettman MA. Thrombolysis of peripheral vascular occlusions with urokinase. A review of the literature. Semin Intervent Radiol. 1992;9:159–165.
- Keen RR, McCarthy WJ, Shireman PK, et al. Surgical management of atheroembolization. J Vasc Surg. 1995;21: 773–781.
- Kent KC, McArdle CR, Kennedy B, et al. A prospective study of the clinical outcome of femoral pseudoaneurysms and arteriovenous fistulas induced by arterial puncture. J Vasc Surg. 1993;17:125–133.
- Kessel DO, Robertson I, Patel JV, et al. Angiographic strategies when iodinated contrast medium is undesirable. Imaging. 2001;13:349–356.
- Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease. JAMA. 2009;295:536–546.
- Kidawa AS. Vasospastic disorders. Clin Podiatr Med Surg. 1992;9:139–150.
- Kinney TB, Rose SC. Intraarterial pressure measurements during angiographic evaluation of peripheral vascular disease: techniques, interpretation, applications, and limitations. Am J Roentgenol. 1996;166:277–284.
- Kitaoka HB, Patzer GL. Arthrodesis for treatment of arthrosis of the ankle and osteonecrosis of the talus. J Bone Joint Surg. 1998;80A:370–379.
- Klein WM, Schlejen PM, Eikelboom BC, et al. MR angiography of the lower extremities with a moving-bed infusion-tracking technique. Cardiovasc Interven Radiol. 2003;26: 1–8.
- Koelemay MJ, den Hartog D, Prins MH, et al. Diagnosis of arterial disease of the lower extremities with duplex ultrasonography. Br J Surg. 1996;83:404–409.
- Köhler A. Ueber eine haufige bisher anscheinende unbekannte Erbronkung einzelner Kurdlicher knocken. Munchen Med Wochenschr. 1908;55:1923.
- Köhler TR, Nance DR, Cramer MM, et al. Duplex scanning for diagnoses of aortoiliac and femoro-popliteal disease. A prospective study. Circulation. 1987;76:1074–1080.
- Köhler C, Weishaupt D, Guggenheim M, et al. Magnetic resonance angiography versus conventional angiography for the planning of reconstructive surgeries. Eur J Trauma Emerg Surg. 2007;33:40–45.
- Kumpe DA, Zwerdlinger S, Griffin DJ. Blue toe syndrome. Treatment with percutaneous transluminal angioplasty. Radiol. 1988;166:37–44.
- Kwasnick AM. Limb salvage in diabetics. Challenges and solutions. Surg Clin North Am. 1986;66:305–318.
- Kwong PK. Applications of Doppler ultrasound to foot and ankle care. Foot Ankle. 1982;2:220–223.
- Lai TM, Glasson R, Grayndler V, et al. Color duplex ultrasonography versus angiography in the diagnosis of lower extremity arterial disease. Cardiovasc Surg. 1996;4:384–388.
- Lapeyre AC III, Steele PM, Kazmier FJ, et al. Systemic embolism in chronic left ventricular aneurysm. Incidence and the role of anticoagulation. J Am Coll Cardiol. 1985;6: 534–538.
- Lapeyre M, Kobeiter H, Desgranges P, et al. Assessment of critical limb ischemia in patients with diabetes: comparison of MR angiography and digital subtraction angiography. Am J Roentgenol. 2005;185:1641–1650.
- Lawrance PF, Syverud JB, Disbro MA, et al. Evaluation of technetium-99m phosphate imaging for predicting skin ulcer healing. Am J Surg. 1983;146:746–750.
- Lecouvet FE, VandeBerg BC, Maldaque BE, et al. Early irreversible osteonecrosis versus transient lesions of the femoral condyles. Prognostic value of subchondral bone and marrow changes on MR imaging. Am J Roentgenol. 1998;170:71–77.
- Leeson MC, Weiner DS. Osteochondrosis of the tarsal cuneiforms. Clin Orthop. 1985;196:260–264.
- Leng GC, Whyman MR, Donnan PT, et al. Accuracy and reproducibility of duplex ultrasonography in grading femoropopliteal stenoses. J Vasc Surg. 1993;17:510–517.
- Levy LA. Foot and ankle ulcers associated with hematologic disorders. Clin Podiatr. 1985;2:631–637.
- Lionberger DR, Bishop JO, Tullos HS. The modified Blair fusion. Foot Ankle. 1982;3:60–62.
- Logerfo FW, Coffmann JD. Vascular and microvascular disease of the foot in diabetics. N Engl J Med. 1984;311: 1615–1619.
- Londrey GL, Ramsey DE, Hodgson KJ, et al. Infrapopliteal bypass for severe ischemia. Comparison of autogenous veins, composite and prosthetic grafts. J Vasc Surg. 1991; 13:631.
- Lovett JE, Shestak KC, Makaroun, MS. Analysis of lower extremity bloodflow in the patient with peripheral vascular insufficiency. A guide for plastic surgeons. Ann Plast Sur. 1994;32:101–106.
- Lynn RB. Chilblains. Surg Gynecol Obstet. 1954;99:720–726.
- Macedo TA, Johnson CM, Hallett JW Jr, et al. Popliteal artery entrapment syndrome: role of imaging in the diagnosis. Am J Roentgenol. 2003;181:1259–1265.
- Maceira E, Rocher R. Müller-Weiss disease: clinical and biomechanical features. Foot Ankle Clin N Am. 2004;9: 105–125.
- Malone JM, Lecel JM, Moore WS. The gold standard for amputation level selection. Xenon-133 clearance. J Surg Res. 1981;30:449–455.
- Mann RA. AVN of the metatarsal head following chevron osteotomy. Foot Ankle. 1982;3:125–129.
- Manusov EG, Lillegard WA, Raspa RF, et al. Evaluation of pediatric foot problems. Am Fam Physician. 1996;54:592–606.
- Marks J, King TA, Baele H, et al. Popliteal-to-distal bypass for limb-threatening ischemia. J Vasc Surg. 1992;15:755.
- Martin ML, Tay KH, Flak B, et al. Multidetector CT angiography of the aortoiliac system and lower extremities: a prospective comparison with digital subtraction angiography. Am J Roentgenol. 2003;180:1085–1091.
- McCauley GK, Koplin PC. Osteochondritis of the tarsal navicular. Radiology. 1977;123:705–706.
- McDermott MM. The magnitude of the problem of peripheral arterial disease: epidemiology and clinical significance. Cleveland Clin J Med. 2006;73(suppl):S2–S7.
- McGhee SR, Boyko EJ. Physical examination and chronic lower extremity ischemia. Arch Intern Med. 1998;158: 1357–1364.
- McNamara TO. Technique and results of higher-dose infusion. Cardiovasc Intervent Radiol. 1998;11:S48–S57.
- McNamara TO, Fischer JR. Thrombolysis of peripheral arterial and graft occlusions: improved results using high-dose urokinase. Am J Roentgenol. 1985;144:769–775.
- Merino J, Casaneuva B, Piney E, et al. Hemiplegia and peripheral gangrene secondary to large and medium sized vessel involvement in CREST syndrome. Clin Rheumatol. 1982;1:295–299.
- Meyerovitz MF, Goldhaber SZ, Reagan K. Recombinant tissue-type plasminogen activators versus urokinase in peripheral arterial and graft occlusions: a randomized trial. Radiology. 1990;175:75–78.
- Milne WK, Worster A. Does clinical examination predict lower extremity peripheral arterial disease. Ann Emerg Med. 2008;12:1–3.
- Mirra JM, Bullough PG, Marcove RC, et al. Malignant fibrous histiocytoma and osteosarcoma in association with bone infarcts. J Bone Joint Surg Am. 1974;56: 932–940.
- Moldveen-Gernimus M, Merrian JC. Cholesterol embolization. From pathologic curiosity to clinical entity. Circulation. 1967;35:946–953.
- Molos MA, Hall JC. Symmetrical gangrene and disseminated intravascular coagulation. Arch Dermatol. 1985;121: 1057–1061.
- Mont MA, Schon LC, Hungerford MW, et al. Avascular necrosis of the talus treated by core decompression. J Bone J Surg. 1996;78B:827–830.
- Moshiak A, Soroker D, Pasik S, et al. Phenol lumbar sympathetic block in diabetic lower limb ischemia. J Cardiovasc Risk. 1995;2:467–469.
- Mubarak SJ. Osteochondrosis of the lateral cuneiform: another cause of a limp in a child. J Bone J Surg. 1992;74A: 285–289.
- Mulfinger GL, Trueta JC. The blood supply of the talus. J Bone Joint Surg. 1970;52B:150–167.
- Muradin GRS, Bosch JL, Verstijnen ACM, et al. Reporting success rate at 12 months after percutaneous treatment of peripheral arterial disease: the impact of outcome criteria. Am J Roentgenol. 2005;185:46–50.
- Nelson JP. The vascular history and physical examination. Clin Podiatr Med Surg. 1992;9:1–18.
- Ogata K, Sugioka Y, Urano U, et al. Idiopathic osteonecrosis of the first metatarsal sesamoid. Skeletal Radiol. 1986; 15:141–145.
- Ohta T. Non-invasive technique using thallium-201 for predicting ischemic ulcer healing in the foot. Br J Surg. 1985; 72:892–895.
- Olin JW, Shih A. Thromboangiitis obliterans (Buerger’s disease). Curr Opin Rheumatol. 2006;18:18–24.
- O’Mara CS, Flinn WR, Neiman HL, et al. Correlation of foot arterial anatomy with early tibial bypass patency. Surgery. 1981;89:743–752.
- Ota H, Takase K, Igarashi K, et al. MDCT compared with digital subtraction angiography for assessment of lower extremity arterial occlusive disease: importance of reviewing cross-sectional images. Am J Roentgenol. 2004;182: 201–209.
- Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery in initial treatment for acute arterial occlusion of the legs. N Engl J Med. 1998; 338:1105–1111.
- Ozonoff MB. Pediatric Orthopedic Radiology. 2nd ed. Philadelphia, PA: WB Saunders; 1992.
- Panayiotopoulos YP, Tyrrell MR, Arnold FJ, et al. Results and cost analysis of distal (crural/pedal) arterial revascularization for limb salvage in diabetic and non-diabetic patients. Diabet Med. 1997;14:214–220.
- Penny JN, Davis LA. Fractures and dislocations of the neck of the talus. J Trauma. 1980;20:1029–1037.
- Perler BA, Osterman FA, Mitchell SE, et al. Balloon dilatation versus surgical revision of infra-inguinal autogenous vein graft stenoses: long-term follow-up. J Cardiovasc Surg. 1990;1:656–661.
- Peterson L, Goldie IF. The arterial supply of the talus. A study on the relationship of experimental talar fractures. Acta Orthop Scand. 1975;46:1026–1034.
- Peterson JJ, Kransdorf MJ, Bancroft LW, et al. Imaging characteristics of cystic adventitial disease of the peripheral arteries: presentation as a soft-tissue mass. Am J Roentgenol. 2003;180:621–625.
- Polak JF, Donaldson MC, Dobkin GR, et al. Early detection of saphenous vein arterial bypass graft stenosis by color-assisted duplex sonography. A prospective study. Am J Roentgenol. 1990;154:857–861.
- Polak JF, Karmel MI, Mannick JA, et al. Determination of the extent of lower-extremity peripheral arterial disease with color assisted duplex sonography: comparison with angiography. Am J Roentgenol. 1990;155:1085–1089.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:527–534.
- Poschenrieder F, Hamer OW, Herold T, et al. Diagnostic accuracy of intraarterial and IV MR angiography for detection of stenoses of the infrainguinal arteries. Am J Roentgenol. 2009;192:117–121.
- Quinn SF, Sheley RC, Semonsen KG, et al. Aortic and lower-extremity arterial disease: evaluation with MR angiography versus conventional angiography. Radiology. 1998;206: 693–701.
- Ramirez G, O Neill WM Jr, Lambert R, et al. Cholesterol embolization. A complication of angiography. Arch Intern Med. 1978;138:1430–1432.
- Ramirez CC, Kirsner RS. A refractory case of erythromelalgia involving the ears. Am J Otolarnygol. 2004;25:251–254.
- Renander A. Two cases of typical osteochondropathy of the medial sesamoid bone of the first metatarsal. Acta Radiol. 1924;3:521–527.
- Resnick D, Kransdorf MJ. Bone and Joint Imaging. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2005.
- Resnick D, Pineda C, Trudell D. Widespread osteonecrosis of the foot in systemic lupus erythematosis. Radiologic and gross pathologic correlation. Skeletal Radiol. 1985;13: 33–38.
- Rhodes GR, Skudder P. Salvage of ischemic diabetic feet. Role of transcutaneous oxygen mapping and multiple configurations of in situ bypass. Am J Surg. 1986;152:165–171.
- Rieger J, Sitter T, Toepfer M, et al. Gadolinium as an alternative contrast agent for diagnostic interventional angiographic procedures in patients with impaired renal function. Nephrol Dial Transplant. 2002;17:824–828.
- Rockson SG, Cooke JP. Peripheral arterial insufficiency. Mechanisms, natural history and therapeutic options. Adv Intern Med. 1998;43:253–277.
- Rosenberg ZS, Beltran J, Bencardino JT. MR imaging of the foot and ankle. RadioGraphics. 2000;20:S153–S179.
- Rubin GD, Schidt AJ, Logan LJ, et al. Multi-detector row CT angiography of the lower extremity arterial inflow and runoff: initial experience. Radiol. 2001;221:146–158.
- Rubinger D, Friedlaender MM, Silver J, et al. Progressive vascular calcification with necrosis of the extremities in hemodialysis patients. A possible role of iron overload. Am J Kidney Dis. 1986;7:125–129.
- Saab MH, Smith DC, Aka PK, et al. Percutaneous transluminal angioplasty of tibial arteries for limb salvage. Cardiovasc Intervent Radiol. 1992;15:211–16.
- Sacks D, Bettmann MA Casciani T, et al. ACR appropriateness criteria for claudication. Revised; 2005, available at: http://www.acr.org/ac.
- Sangeorzan BJ, Benirschke DK, Mosca V, et al. Displaced intra-articular fractures of the tarsal navicular. J Bone Joint Surg. 1989;71A:1504–1510.
- Scaglietti O, Struga G, Mizzore W. Plus-variant of the astragalus and subnormal scaphoid space. Two important findings in Koehler’s scaphoid necrosis. Acta Orthop Scand. 1962;32:499–508.
- Schernthaner R, Fleischmann D, Stadler A, et al. Value of MDCT angiography in developing treatment strategies for critical limb ischemia. Am J Roentgenol. 2009;192: 1416–1424.
- Schwarten DE. Clinical and anatomical considerations for non-operative therapy in tibial disease and the results of angioplasty. Circulation. 1991;83(suppl 1):186–190.
- Seabrook GR, Mewissen MW, Schmitt DD, et al. Percutaneous intra-arterial thrombolysis in the treatment of thrombosis of lower extremity arterial reconstructions. J Vasc Surg. 1991;13:646–651.
- Sever JW. Apophysitis of the os calcis. N Y Med J. 1912;95: 1025–1029.
- Shafa MH, Fernandez-Ulloa M, Rost RC, et al. Diagnosis of asceptic necrosis of the talus by bone scintigraphy. Clin Nucl Med. 1983;8:50–53.
- Sharafuddin MJ, Hicks ME. Current status of percutaneous mechanical thrombectomy. Part III. Present and future applications. J Vasc Interv Radiol. 1998;9:209–224.
- Sharafuddin MJ, Hicks ME. Current status of percutaneous mechanical thrombectomy. Part II. Devices and mechanisms of action. J Vasc Interv Radiol. 1998;9:15–31.
- Sharafuddin MJ, Hicks ME. Current status of percutaneous mechanical thrombectomy. Part I. General principles. J Vasc Interv Radiol. 1997;8:911–921.
- Shofner CE, Coin CG. The effect of weight bearing on the appearance and development of the secondary calcaneal apophysis. Radiology. 1966;86:201–206.
- Si T-G, Guo Z, Hao X-S. Can catheter-directed thrombolysis be applied to acute lower extremity artery embolism after recent cerebral embolism from atrial fibrillation? Clin Radiol. 2008;63:1136–1141.
- Siegel ME, Stewart CA, Kwong P, et al. 201 Tl perfusion study of ischemic ulcers of the leg. Prognostic ability compared with Doppler ultrasound. Int J Nucl Med Biol. 1982; 143:233–235.
- Siffert RS. Classification of osteochondroses. Clin Orthop. 1981;158:10–28.
- Siffert RS, Arkin AM. Post-traumatic aseptic necrosis of the distal tibial epiphysis. J Bone Joint Surg. 1950;32A:691–695.
- Simon TD, Soep JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:e472–e475.
- Sivananthan UM, Browne TF, Thorley DJ, et al. Percutaneous transluminal angioplasty of the tibial arteries. Br J Surg. 1994;81:1282–1285.
- Smillie IS. Freiberg’s infarction (Köhler’s second disease). J Bone Joint Surg. 1957;39B:580.
- Smillie IS. Treatment of Freiberg’s infraction. Proc Royal Soc Med. 1967;60:29–31.
- Soulen RL, Tyson RR, Reichle FA, et al. Angiographic criteria for small vessel bypass. Radiology. 1973;107:513–519.
- Spies JB. Complications of diagnostic arteriography. Semin Intervent Radiol. 1994;2:93–101.
- Spinosa DJ, Matsumoto AH, Angle JF, et al. Safety of CO2 and gadodiamide-enhanced angiography for evaluation of percutaneous treatment of renal artery stenosis in patients with chronic renal insufficiency. Am J Roentgenol. 2001; 176:1305–1311.
- Spinosa DJ, Matsumoto AH, Hagspiel KD, et al. Gadolinium-based contrast agents in angiography and interventional radiology. Am J Roentgenol. 1999;173:1403–1409.
- Stillman RM. Infrainguinal occlusive disease. E-medicine. June 24, 2008. Available at: http://emedicine.medscape.com.
- Strozik K. Case of primary erythromelalgia in a child. Clin Pediatr. 1992;31:378–379.
- Tatli S, Lipton MJ, Davison BD, et al. MR imaging of aortic and peripheral vascular disease. RadioGraphics. 2003;23: S59–S78.
- Terada M, Satoh M, Mitsuzane K, et al. Short-term intrathrombotic injection of ultrahigh-dose urokinase for treatment of iliac and femoropopliteal artery occlusions. Radiat Med. 1990;8:79–87.
- Thornton MA, Gruentzig AR, Hallman J, et al. Coumadin and aspirin in prevention of recurrence after transluminal coronary angioplasty. A randomized study. Circulation. 1984;69:721–727.
- Valji K, Roberts A, Davis GB, et al. Pulsed-spray thrombolysis of arterial and bypass graft occlusion. Am J Roentgenol. 1991;156:617–621.
- van Breda A, Katzen BT. Thrombolytic therapy of peripheral vascular disease. Semin Intervent Radiol. 1995;2:354–366.
- Varty K, Bolia A, Naylor AR, et al. Infrapopliteal percutaneous transluminal angioplasty. A safe and successful procedure. Eur J Vasc Endovasc Surg. 1995;9:341–345.
- Veith FJ, Gupta SK, Wengerter KR, et al. Changing arterosclerotic disease patterns and management strategies in lower limb threatening ischemia. Ann Surg. 1990; 212:402–412.
- Wagner HJ, Starck EE, Reuter P. Long-term results of percutaneous aspiration embolectomy. Cardiovasc Intervent Radiol. 1994;17:241–246.
- Wang GJ, Sweet DE, Roger SI. Fat cell changes as a mechanism of avascular necrosis of the femoral head in corticosteroid treated rabbits. J Bone Joint Surg. 1977;59A: 729–735.
- Wang Y, Lee HM, Khilnani NM, et al. Bolus-chase MR digital subtraction angiography of the lower extremity. Radiology. 1998;207:263–269.
- Washburn B. Frostbite. N Engl J Med. 1962;266:974–989.
- Weaver FA, Modrall JG, Baek S, et al. Syme amputation. results in patients with severe forefoot ischemia. Cardiovasc Surg. 1996;4:81–86.
- Wengerter KR, Veith FJ, Gupta SK, et al. Prospective randomized multicenter comparison of in situ and reversed vein infra-popliteal bypasses. J Vasc Surg. 1991;13: 189–197.
- Willmann JK, Baumert B, Schertler T, et al. Aortoiliac and lower extremity arteries assessed with 16-detector row CT angiography: prospective comparison with digital subtraction angiography. Radiology. 2005;236: 1083–1093.
- Wilson YG, Wyatt MG, Currie IC, et al. Isolated tibial vessel disease. treatment options and outcome. Panminerva Med. 1996;38:71–77.
- Wright DG, Adeloar RS. Avascular necrosis of the talus. Foot Ankle Int. 1995;16:743–744.
- Yu J, Tanner J. Considerations in metatarsalgia and midfoot pain: an MR imaging perspective. Semin Musculoskel Radiol. 2002;6:91–104.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree