PART IX Spine Vascular Interventions
A 63-year-old man presents with a 6-month history of progressive gait disturbance, weakness and diffuse paresthesias in his legs, and low back pain. MRI is performed.
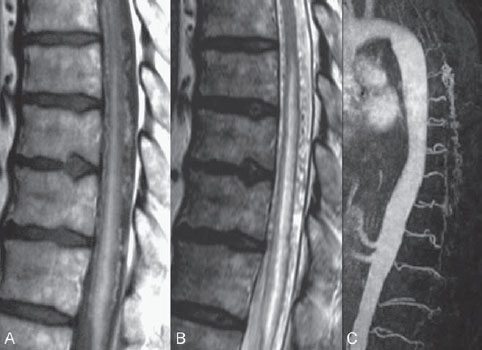
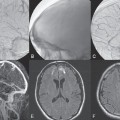
Fig. 60.1 (A) Sagittal T1-weighted contrast-enhanced and (B) T2-weighted MRI demonstrates dilated perimedullary vessels along the posterior surface of the cord. Evidence of cord edema with contrast enhancement is noted, suggestive of venous congestion. (C) Contrast-enhanced MRA during the arterial phase shows early filling of venous structures corresponding to an AV shunt.
MRI
MRI demonstrated pathologically dilated perimedullary vessels along the posterior and, to a lesser degree, anterior surfaces of the cord on T2-weighted scans. In addition, evidence of cord edema was noted on T2-weighted scans. Following contrast enhancement, the pathologic vessels were detected again, and there was evidence of contrast enhancement within the cord. Contrast-enhanced MRA demonstrated filling of venous structures in the early arterial phase, indicating an arteriovenous (AV) shunt. No pathologic vessels were seen within the cord parenchyma (Fig. 60.1).
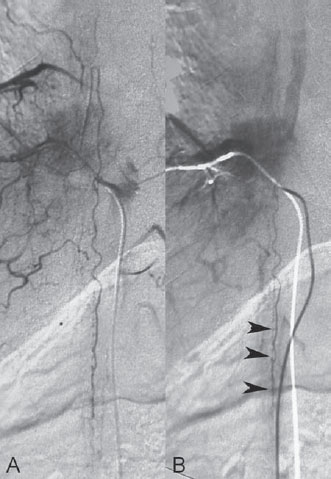
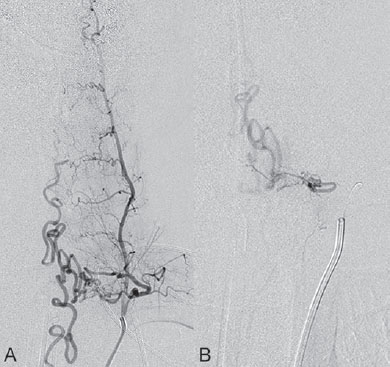
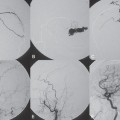
Fig. 60.2 DSA. Injection into the segmental artery from which the ASA originates in AP view in (A) arterial and (B) venous phases demonstrates stagnation of contrast within the ASA (arrowheads).
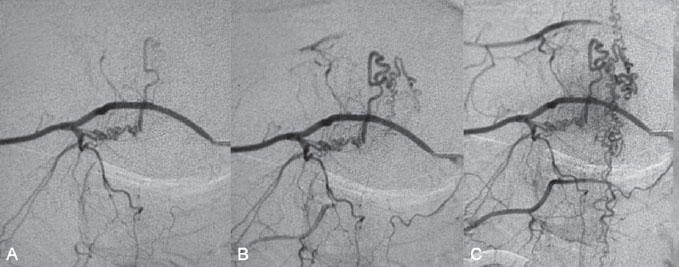
After injection into the segmental artery from which the anterior spinal artery (ASA) originated, stagnation of contrast in the ASA was observed until late in the venous phase, indicating interference with the circulation that was likely caused by venous hyperpressure (Fig. 60.2). Following injection into the right T10 segmental artery, the shunt was identified, originating from a radiculomeningeal artery and filling in retrograde fashion a radicular vein that ascended toward the perimedullary veins (Fig. 60.3).
Fig. 60.3 DSA. Right T10 segmental artery angiogram in AP view reveals a DAV shunt from a radiculomeningeal artery to perimedullary veins in the (A,B) early and (C) late arterial phases.
Spinal dural arteriovenous fistula (DAVF) with venous congestion
EQUIPMENT
- Standard 5F access (puncture needle, 5F vascular sheath)
- 5F Cobra catheter with continuous flush and a 0.035-in hydrophilic guidewire
- A 0.012-in flow-directed microcatheter (Magic; Balt International, Montmorency, France) with a 0.008-in guidewire (Mirage; ev3, Plymouth, MN)
- A 10% glucose solution
- Histoacryl/Lipiodol (0.5 mL/1.5 mL)
- Contrast material
DESCRIPTION
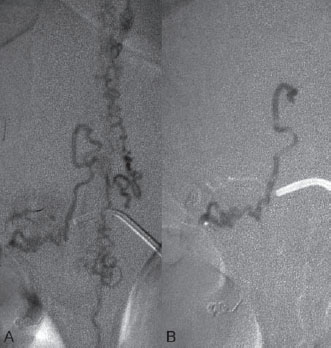
A 5F Cobra catheter was placed distally into the segmental artery with the aid of a guidewire, which was placed far distally to ensure stability when the guiding catheter was pushed. Once stable access was achieved, a roadmap was performed, and a flow-directed microcatheter with a steam-shaped 45-degree curve was introduced into the segmental artery and advanced as far distally toward the shunting zone as possible with the aid of a micro-guidewire. Here, microcatheter injections demonstrated a shunting zone underneath the pedicle of the T10 vertebral body. After the microcatheter had been flushed with glucose solution, a mixture of 0.5 mL of glue with 1.5 mL of Lipiodol was slowly injected that penetrated through the fistulous zone into the vein, thereby occluding the distal arterial segment, the proximal vein, and the intervening shunting zone (Fig. 60.4).
Fig. 60.4 (A) Superselective microcatheter injection and (B) glue cast following embolization show penetration of the glue through the fistulous zone into the proximal vein.
Spinal DAVFs are the most frequent vascular malformation of the spine, accounting for ~70% of all vascular malformations of the spine. Spinal DAVFs tend to affect elderly men. Most fistulae are solitary lesions and found in the thoracolumbar region. Spinal DAVFs are acquired, although the exact etiology is not known. The AV shunt is usually located at the level of the dura close to the spinal nerve root, where blood from the radiculomeningeal artery (i.e., the artery that supplies the nerve root and meninges but not necessarily the spinal cord!) enters a radicular vein; the latter passes the dura at the dorsal surface of the dural root sleeve in the intervertebral foramen. The transition is classically located directly underneath the pedicle of the vertebral body supplied by the injected segmental artery. This location is the most frequent, but shunts may occur in other locations along the dura between the nerve roots. Supply to shunts in these locations is from the adjacent segmental levels, so that dual supply is common. The increase in spinal venous pressure due to arterialization diminishes the AV pressure gradient and leads to decreased drainage of the normal spinal cord veins with venous congestion, which results in intramedullary edema. This in turn causes chronic hypoxia and progressive myelopathy.
PHYSICAL EXAMINATION
- Initial symptoms of venous congestion of the cord are nonspecific and include difficulty climbing stairs and gait disturbances. More often, sensory symptoms such as paresthesias, diffuse or patchy areas of sensory loss, and radicular pain may affect both lower limbs or, initially, one limb. Lower back pain without a radicular distribution is also frequently encountered. These neurologic symptoms are progressive and often ascending. Bowel and bladder incontinence, erectile dysfunction, and urinary retention are more often seen late in the course of the disease. These symptoms are invariably progressive.
CT/CTA
- The initial diagnosis of a dural AV shunt cannot be made on unenhanced CT. Spinal CTA may demonstrate enlarged vessels but is rarely used for screening. Because DAVFs can be present from the level of the foramen magnum to the sacral region, the radiation burden for CTA is unnecessarily high, given the better MRA options for visualizing the origin of a shunt.
MRI/MRA
- In our experience, the diagnosis is often strongly suspected by MRI and confirmed by contrast-enhanced MRI. On T2-weighted sequences, cord edema is depicted over multiple segments; this is often accompanied by a hypointense rim, most likely representing deoxygenated blood within the dilated capillary vessels surrounding the region of congestive edema. Further in the course of the disease, the cord will become atrophic.
- The perimedullary vessels are dilated and can be observed on T2-weighted images as flow voids, which are often more pronounced on the dorsal surface than on the ventral surface. However, if the shunt volume is small, they may be seen only after contrast enhancement. The serpiginous vascular structures may be better appreciated on heavily T2-weighted sequences (CISS [constructive interference in steady state], FIESTA [fast imaging employing steady-state acquisition], or 3D TSE [turbo spin echo]) than on standard T2 TSE sequences. In addition, these sequences may be useful to differentiate pulsation artifacts, which are sometimes mistaken for flow voids, from true vascular structures.
- Neither the location of the pathologic vessels nor the intramedullary imaging findings seem to be related to the actual level of the fistula.
- On T1-weighted scans, the swollen cord is slightly hypointense and enlarged. Following the administration of contrast, diffuse enhancement may be seen within the cord as a sign of chronic venous congestion with breakdown of the blood-spinal cord barrier.
- Spinal DAVFs may occur anywhere from the level of the foramen magnum to the sacrum, and the localization of these lesions can be difficult and challenging, especially in cases in which the cord edema is distant from the AV shunt. Thus, a noninvasive evaluation of the shunt location is extremely helpful to guide invasive catheter angiography.
- Contrast-enhanced MRA of the spine has greatly contributed to the localization of these lesions and helps avoid unnecessary superselective injections of all possible arterial feeders. The technique of first-pass gadolinium-enhanced MRA can clearly demonstrate early venous filling, thereby confirming the presence of a shunt. In most cases, it can also demonstrate the level of the shunt.
- On selective angiography, stasis of contrast material in the radiculomedullary arteries, especially the ASA, can be seen. Delayed venous return following an ASA injection indicates venous congestion and the need to search for a shunting lesion, whereas normal venous return following injection of the ASA will in most cases exclude the possibility of a spinal DAVF.
- After injection into the segmental artery harboring an AVF, early venous filling and the retrograde uptake of contrast in the radiculomedullary veins are visualized. Often, an extensive network of dilated perimedullary veins is visible. This network may even recruit supply from dural arteries that ascend or descend from neighboring radiculomeningeal arteries.
- The shunting zone is frequently located underneath the pedicle of the injected segmental artery where the radiculomeningeal artery pierces the dura.
- In rare cases, the flow from the radiculomeningeal artery into the radicular vein may be slow; therefore, we classically perform spinal angiography to search for DAVFs with a slow frame rate (one image per second) and wait for at least 4 seconds to exclude delayed retrograde filling of the radicular veins.
- The clinical differential diagnosis of entities with the rather unspecific neurologic symptoms is manifold and includes polyneuropathy, tumor, and degenerative disk disease. Urinary retention may be misinterpreted as related to prostrate hypertrophy.
- The dual MRI findings of cord edema and dilated perimedullary vessels without any intramedullary nidus of vessels are typical of spinal DAVF. However, the findings in reality are nonspecific and can also be seen with epidural and paraspinal AV shunts, which are characterized by retrograde drainage toward the perimedullary venous system of the cord as well as vascular lesions of the spinal cord, such as multiple hemangioblastomas.
- A spinal DAVF that drains solely into the anterior spinal veins may only demonstrate cord hyper-signal on T2-weighted images because the anterior spinal veins have a subpial location and may therefore not be visualized as dilated. In these cases, a glioma (especially when contrast uptake is present), an inflammatory lesion, or spinal ischemia must be included in the differential diagnosis.
- On DSA, radicular arteriovenous malformations (rAVMs), epidural AV shunts, and perimedullary AVFs must be differentiated; rAVMs or AVMs of the nerves usually have abnormal vessels that form a nidus surrounding the nerve root, whereas spinal DAVFs have an apparent shunt zone with radicular feeders converging into the same draining vein. These patients often present with radicular pain, not direct symptoms of congestive venous myelopathy. Epidural AV shunts are located in the epidural space and normally recruit supply from the vertebral body and surrounding structures, with drainage directly into the epidural plexus and not necessarily a perimedullary vein. Symptoms are related to compression of the adjacent nerve root or cord rather than venous congestion, unless unusual cases of perimedullary reflux occur. Perimedullary AVFs, including fistulae of the filum terminale, are flow shunts at the surface of the cord (or the filum) that are invariably supplied by arteries that in normal circumstances would supply the cord (i.e., the radiculomedullary arteries forming the ASA or the radiculopial arteries via the posterior spinal artery).
SURGICAL TREATMENT
- Surgery is aimed at identifying and obliterating the fistulous point, which most of the time is underneath the pedicle of the injected segmental artery from which the supply to the fistula was visualized.
- Infrequently, the dural shunt is located between segmental levels, and the leptomeningeal vein enters the dura by itself between levels.
- A hypervascularized network can be present within the dura that opens into the (intradural) radicular vein. Disconnection of the vein with or without coagulation of the arterialized network within the dura can be performed to treat the condition surgically.
ENDOVASCULAR TREATMENT
- In our practice, embolization with liquid material (diluted glue) is the therapy of choice.
- The embolic material must reach the proximal part of the vein.
- If the supply to the spinal cord is seen to arise from the same segmental level, endovascular therapies will be associated with a higher risk for neurologic deterioration if the spinal cord supply is inadvertently occluded, and surgical treatment strategies will be preferable.
- Postprocedural heparin to prevent excessive venous thrombosis may be recommended if significant passage of the liquid embolic agent occurs.
- Failure to penetrate the proximal vein will likely result in delayed reopening of the fistula (see Case 61).
- To avoid reflux into an artery supplying the spinal cord, a careful evaluation is necessary to determine the presence of vessels supplying the spinal cord from the segmental artery to be injected, from the same level on the opposite side, and from the segmental arteries above and below the injected pedicle.
- Far distal migration of the embolic agent into the draining vein can cause symptoms to worsen and must be avoided.
Published Literature on Treatment Options
There are two options in the treatment of spinal DAVFs: surgical disconnection of the leptomenigeal vein that receives the blood from the shunt zone (a relatively simple and safe intervention) and endovascular therapy with a liquid embolic agent after superselective catheterization of the feeding radiculomeningeal artery. The embolic agent must reach the nidus and occlude the proximal segment of the draining vein to prevent recurrence of the fistula. The success rates of endovascular therapy have been reported to vary between 25 and 75%, whereas a recent meta-analysis suggested that the rate of complete occlusion of a fistula following surgery is 98%. Because DSA is necessary to confirm the diagnosis, the treatment strategy that is adopted by most centers nowadays includes a tentative embolization if this is felt to be a safe (i.e., no artery supplying the spinal cord arises from the same pedicle as the shunt feeder). If the liquid embolic agent penetrates the vein, long-term clinical follow-up has shown that this results in complete obliteration of the fistula and a good clinical outcome. If the glue remains in an intra-arterial location, the liquid embolic agent may at least be used to identify the feeding artery and so facilitate the intraoperative fluoroscopic localization of the exact level of the fistula.
PEARLS AND PITFALLS__________________________________________________
- Spinal DAVFs are a rare but treatable cause of otherwise progressive paraplegia. The neuroradiologist plays a major role in the detection of these lesions, as well as their treatment.
- The neurologic symptoms are nonspecific, but the MRI triad of cord edema, prominent perimedullary vessels, and contrast enhancement of the cord in elderly men should lead to the diagnosis of a DAV shunt. The exact level of the shunt can be predicted by contrast-enhanced MRI, then confirmed by selective DSA.
- Treatment must be aimed at occluding the proximal portion of the leptomemingeal vein in which reflux is occurring.
Farb RI, Kim JK, Willinsky RA, et al. Spinal dural arteriovenous fistula localization with a technique of first-pass gadolinium-enhanced MR angiography: initial experience. Radiology 2002;222(3):843–850
Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol 2009;30(4):639–648
Van Dijk JM, TerBrugge KG, Willinsky RA, Farb RI, Wallace MC. Multidisciplinary management of spinal dural arteriovenous fistulas: clinical presentation and long-term follow-up in 49 patients. Stroke 2002;33(6):1578–1583
A 41-year-old man presents with a 3-month history of paresthesias and weakness when climbing stairs, and he has been experiencing erectile dysfunction during the past few weeks. MRI demonstrates the classic findings of a spinal dural arteriovenous shunt with dilated perimedullary vessels on T2-weighted images, and MRA shows cord edema and evidence of early vein filling. Angiography with possible embolization is performed.
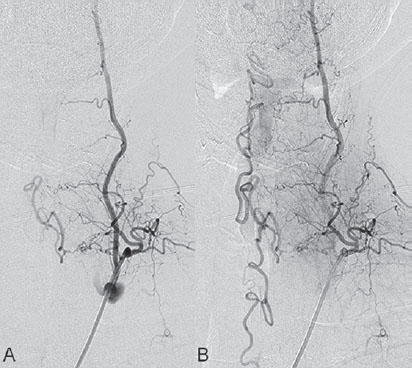
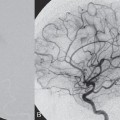
Fig. 61.1 DSA. (A) Left T2 segmental artery angiogram in AP view and (B) superselective microcatheter injection demonstrate a shunt from a radiculomeningeal artery draining into perimedullary veins.
DSA
Injection of the left deep cervical artery demonstrated the T2 segmental artery to be opacifying a shunting zone underneath the pedicle of the left T2 vertebral body. The shunt was fed by the radiculomeningeal artery and drained both cranially and caudally via the radicular vein into the perimedullary veins (Fig. 61.1).
Spinal dural arteriovenous fistula (DAVF) at T2 with venous congestion
EQUIPMENT
- Standard 5F access (puncture needle, 5F vascular sheath)
- 5F Cobra catheter with continuous flush and a 0.035-in hydrophilic guidewire
- A 0.012-in flow-directed microcatheter (Magic; Balt International, Montmorency, France) with a 0.008-in guidewire (Mirage; ev3, Plymouth, MN)
- A 10% glucose solution
- Histoacryl/Lipiodol (0.5 mL/1.5 mL)
- Contrast material
DESCRIPTION
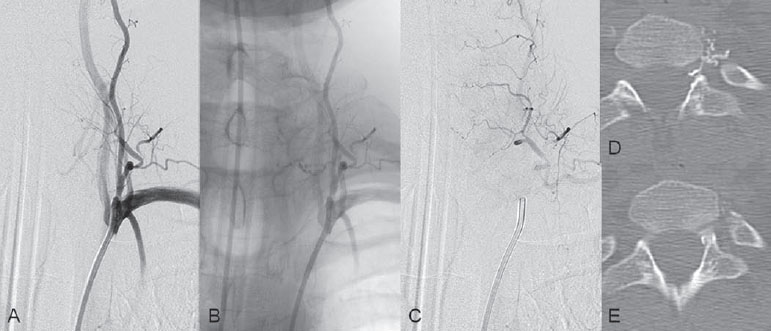
The 5F Cobra catheter was placed distally into the segmental artery, and a flow-directed microcatheter with a steam-shaped 45-degree curve was introduced into the segmental artery and advanced as far distally toward the shunting zone as possible with the aid of a micro-guidewire. Here, microcatheter injections demonstrated the shunt to be in close proximity to the tip of the microcatheter. After the microcatheter had been flushed with glucose solution, a mixture of 0.5 mL of glue with 1.5 mL of Lipiodol was injected that precipitated before entering the proximal vein and remained at the level of the dura. Follow-up angiography demonstrated no filling in the veins; however, given the incomplete venous penetration, which was verified by axial CT scans through the embolized region, the patient was forewarned that his symptoms would likely recur and that surgical intervention would then be necessary (Fig. 61.2). After an initial decrease in his symptoms, the patient again noticed increasing weakness 4 weeks after the embolization procedure. Follow-up angiography demonstrated the predicted reconstitution of the fistula (Fig. 61.3). Subsequent uneventful surgery resulted in complete obliteration of the fistula.
Fig. 61.2 (A) Left deep cervical artery angiogram in AP view after embolization in arterial phase, (B) unsubtracted image, and (C) angiogram in capillary phase following embolization reveal no filling of the veins; however, there is incomplete penetration of the glue cast into the proximal vein, which is also demonstrated on (D,E) unenhanced axial CTs in bone window.
Incomplete occlusion of a shunt because of the failure of venous penetration occurs in 25 to 75% of cases and is typically due to early polymerization of the embolic agent before it reaches the venous site. It is the task of the neurointerventionalist to recognize this rather common technical failure and to arrange for the patient to undergo surgery when clinical symptoms recur. The patient generally experiences a short decrease in symptoms; however, because of good collateralization within the dura, the fistula invariably reopens at some time in the subsequent weeks or months.
PHYSICAL EXAMINATION
- Symptoms may decrease initially because the immediate pressure in the venous system is reduced. This is classically followed after weeks or months by a slow deterioration of the clinical status.
CT/CTA
- If it is questionable whether the liquid embolic material has penetrated the proximal (intradural) vein, unenhanced CT may help to detect the glue cast and its extension toward the vein.
- Because the location of the shunt is known, CTA may prove helpful to evaluate filling of the shunting zone in the arterial phase and reconstitution of the fistula.
MRI/MRA
- Following incomplete embolization of a shunt, MRI may initially demonstrate a reduction in flow voids and even cord edema; however, with reconstitution of the shunt via collaterals (this will invariably happen), findings similar to those on the pre-embolization MR images will be seen, with reappearance of the flow voids and cord edema.
- Contrast-enhanced MRA will visualize reconstitution of the fistula; however, the spatial resolution may not be sufficient to answer the question of whether the same or adjacent feeders are involved in reconstitution of the fistula.
- Immediately after unsuccessful embolization, injection into the segmental artery that harbored the shunt will not demonstrate any residual filling; however, because of the dense collateralization in the dura, a delayed reopening of the shunt will occur.
- Therefore, following incomplete (i.e., proximal) embolization of a spinal dural AV shunt, follow-up angiography must include not only the segmental artery that previously harbored the shunt but also adjacent and contralateral segmental arteries because they may reconstitute the shunting zone via intersegmental dural or paravertebral anastomoses.
- Clinical deterioration following embolization of a spinal DAVF is in most cases related to insufficient venous penetration and reopening of the fistula. However, in patients with long-standing symptoms, these may worsen progressively despite complete occlusion of the fistula because of progressive cord atrophy. However, the metachronous appearance of a secondary DAVF must also be considered.
- MRA may help to identify a shunt (either residual or de novo).
SURGICAL TREATMENT
- Surgery is the method of choice once endovascular treatment options have proved unsuccessful.
- The (proximal) glue cast can help the neurosurgeon identify the correct level before opening the dura and occluding the radicular vein.
ENDOVASCULAR TREATMENT
- Following proximal embolization and reconstitution of the fistula via dural collaterals, a more diffuse dural network is classically present. In addition, supply from adjacent segmental arteries is often seen. In these cases, endovascular treatment options are limited, and surgery should be performed.
- The likelihood of failure to penetrate the proximal vein is significantly increased in reconstituted DAVFs (i.e., shunts that were unsuccessfully treated in the first session).
- To avoid further neurologic deterioration, surgery should be performed without delay.
Published Literature on Treatment Options
The aim of treatment in spinal DAVFs is to occlude the shunting zone (i.e., the most distal part of the artery together with the most proximal part of the draining vein). A proximal arterial occlusion will lead to a transient decrease in symptoms. Because of the good collateralization of the dura, however, the fistula tends to recur within the following months. Therefore, proximal occlusions with coils or Gelfoam are contraindicated. Embolization with particles is also likely to lead to early recanalization and is therefore not indicated. Liquid agents are the embolic materials of choice, and both Onyx and glue can be used. The only caveat is that because of the rich anastomotic network, caution must be taken that the liquid embolic material does not penetrate adjacent segmental arteries, which may be supplying the spinal cord. It is therefore our policy to check the adjacent levels for spinal cord supply before performing liquid embolic material embolization and to stop the injection when the embolic material reaches the adjacent segmental arteries if supply to the cord from these vessels has been visualized. If the embolic agent does not reach the venous site, we strongly advocate early surgical intervention because a recent study has shown that patients with an incomplete endovascular occlusion who subsequently required surgical intervention had a poor clinical outcome, which was likely at least in part due to delay of the secondary intervention.
PEARLS AND PITFALLS__________________________________________________
- A proximal vessel occlusion is not sufficient, despite initial angiographic occlusion, because the DAVF will recruit meningeal collaterals and will be reconstituted within the following weeks or months.
- If it is uncertain that glue has reached the vein, CT may be helpful to demonstrate penetration distal to the dura.
- Neurologic deterioration following embolization of a DAVF may be due to (1) reopening of the fistula, (2) development of a secondary fistula at a different location, or (3) progressive cord atrophy in a patient with long-standing symptoms despite complete occlusion.
Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol 2009;30(4):639–648
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree