CHAPTER 10 A Brief Guide to Cancer Imaging
Cancer imaging constitutes a significant part of the typical abdominal imaging workload of many radiology practices. Familiarity with the modes and patterns of tumor spread can improve interpretive efficiency and accuracy because malignant tumors often present distinct from their origin. Likewise, knowledge of the staging of malignant tumors helps one to convey information to the referring physician that can facilitate tumor resection or prevent unnecessary surgery in patients with advanced disease. For these reasons, this chapter is dedicated to the staging and spread of some common malignant tumors of the abdomen and pelvis.
MECHANISMS OF TUMOR SPREAD
The mechanisms and pathways by which malignant tumors of the abdomen and pelvis spread are determined by regional anatomy and tumor pathophysiology. Abdominal and pelvic organs are suspended in the peritoneal cavity by ligaments and mesenteries formed by the peritoneum as it reflects from the extraperitoneal surface. The abdominal ligaments and mesenteries serve as conduits through which blood vessels, nerves, and lymphatics may travel. They also may serve to facilitate tumor spread between organs or restrict tumor spread between compartments. A variety of descriptions are in common usage regarding the mechanisms of tumor spread. Tumors can spread in several ways: (1) directly from organ to organ without regard for fascial planes or anatomic compartments; (2) via the subserous connective tissue, blood vessels, or lymphatics of the abdominal mesenteries and ligaments; or (3) throughout the peritoneal cavity. Chapter 6 provides additional information regarding the spread of disease within the abdomen and pelvis.
Direct Contiguous Spread
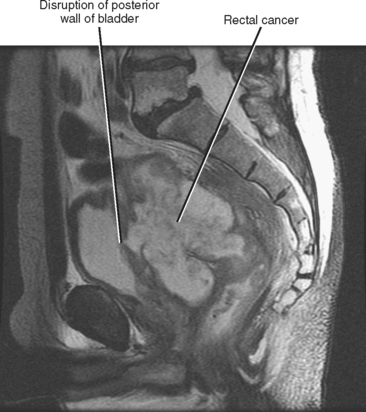
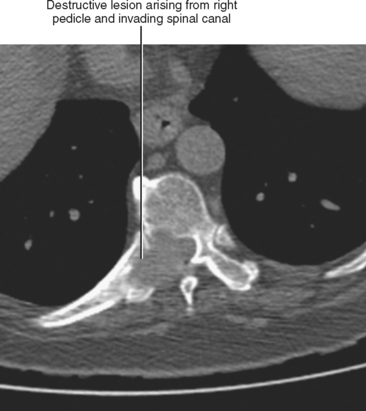
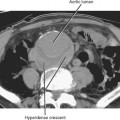
Direct invasion by tumor can occur from the organ of origin to a contiguous organ across fascial planes. This typically occurs with locally aggressive tumors of the kidneys and pelvic organs (e.g., uterus, ovaries, prostate). Examples of this type of spread include invasion of the sigmoid colon by ovarian cancer, bladder invasion by rectal cancer, and rectal invasion by prostate cancer (Figs. 10-1 and 10-2). Similarly, renal cell carcinoma may violate normal anatomic boundaries to directly invade the small bowel or colon. Direct invasion of an organ should be suspected on computed tomography (CT) or magnetic resonance imaging (MRI) when there is loss of a normal fat plane between the tumor and the secondarily involved organ.
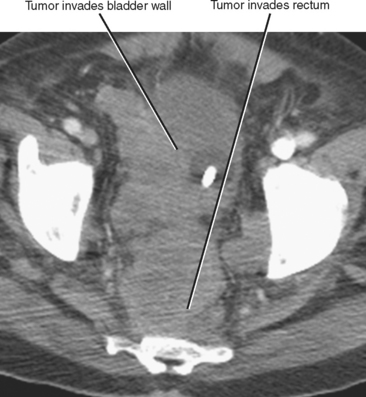
Figure 10-1 Enhanced axial computed tomographic scan in a patient with prostate cancer invading the bladder and rectum.
Subperitoneal Spread
Subperitoneal Tumor Extension
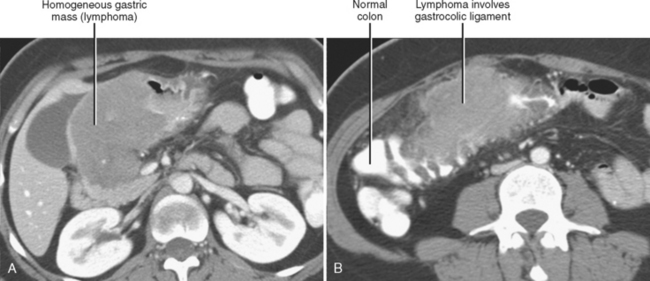
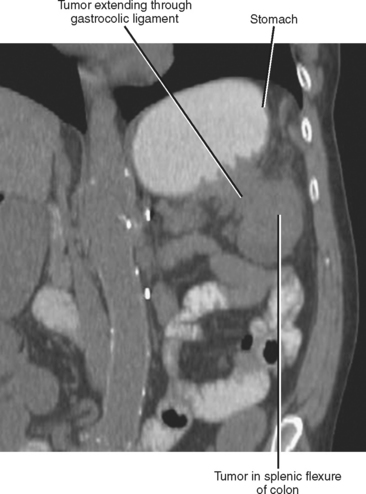
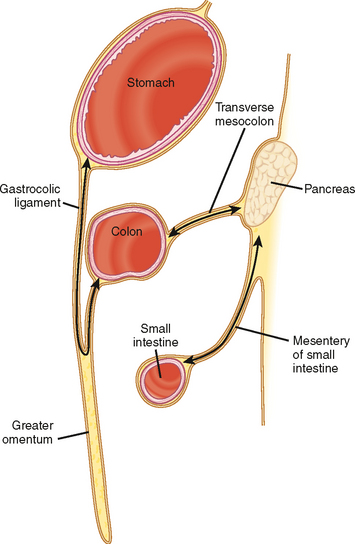
The subperitoneal space provides a bidirectional pathway via which many tumors spread from organ to organ. This network also creates a natural communication interconnecting the extraperitoneum and the suspended intraperitoneal organs. In this manner, for example, malignant tumor may extend through the subserous connective tissues to the transverse colon via the transverse mesocolon from the pancreas or the gastrocolic ligament from the stomach. It is important to remember that spread of tumor may occur in any direction between organs within the subperitoneal space (e.g., colon to stomach or from stomach to colon) (Figs. 10-3 to 10-5).
Lymphatic Spread
LYMPH-NODE ANATOMY AND PATTERNS OF LYMPHATIC SPREAD OF TUMOR
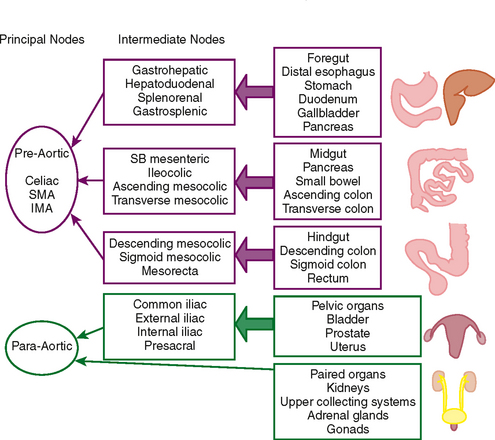
Dissemination of cancer cells via the lymphatic channels to regional and distant lymph nodes constitutes a relatively common form of subperitoneal tumor spread. Knowledge of common lymph-node locations and the lymphatic drainage patterns within the abdomen and pelvis helps guide the search for lymph-node metastases in the setting of abdominal or pelvic cancer (Fig. 10-6).
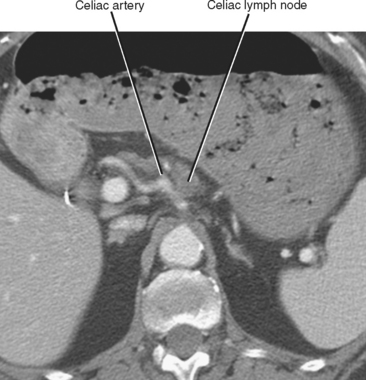
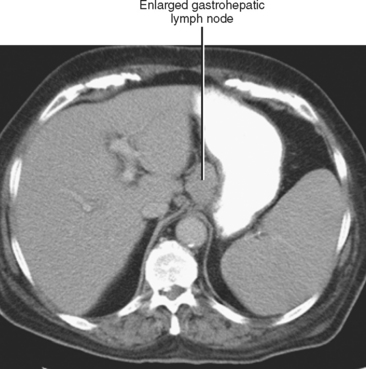
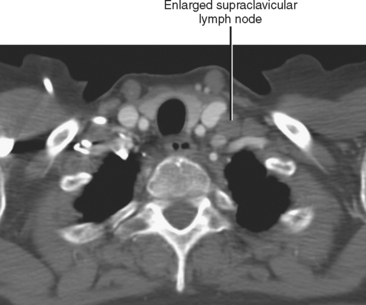
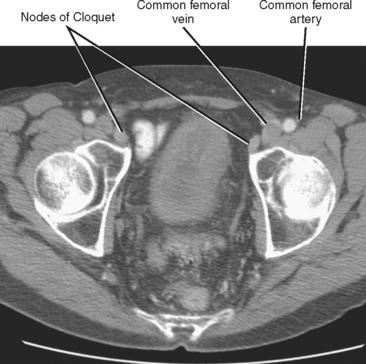
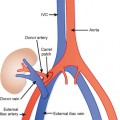
In general, the lymph nodes are distributed along the blood vessels for which they are named. For example, the celiac lymph nodes are near the celiac artery, whereas the external iliac lymph nodes are distributed along the external iliac arteries (Fig. 10-7). Extraperitoneal lymph nodes along the aorta and inferior vena cava (IVC) are named in terms of their location relative to those structures (e.g., retrocaval, paraaortic, preaortic). The preaortic nodes drain the abdominal portion of the gastrointestinal tract and its derivates (e.g., liver, spleen, pancreas) supplied by the three ventral branches of the aorta: the celiac nodes drain the foregut, the superior mesenteric nodes drain the midgut (e.g., small bowel), and the inferior mesenteric nodes drain the hindgut (e.g., sigmoid colon). The paraaortic nodes drain the viscera and structures supplied by the lateral and dorsal branches of the aorta (e.g., kidneys and adrenal glands, gonads, uterus, prostate, bladder, abdominal wall, and lower extremities). A few lymph-node groups are named based on their relation to an anatomic region rather than a vessel (e.g., retrocrural, gastrohepatic, mesorectal, presacral, inguinal) (Figs. 10-8 and 10-9). Two lymph nodes are frequently referred to by their eponyms. Virchow’s node classically refers to a palpable supraclavicular lymph node (typically left) involved with metastatic disease. When an enlarged supraclavicular lymph node is present in the absence of significant chest disease, a search should be conducted for infradiaphragmatic tumor (Fig. 10-10). The node of Cloquet refers to the deepest inguinal lymph node in the femoral canal that is the first lymph node underneath the inguinal ligament (Fig. 10-11). This lymph node serves as a useful anatomic landmark. Lymph nodes central to this level are considered external iliac, whereas lymph nodes below or peripheral to this level are considered inguinal.
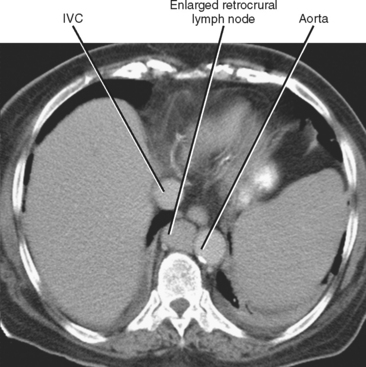
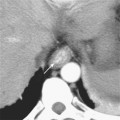
Figure 10-8 Enhanced axial computed tomographic scan shows enlarged retrocrural lymph node. IVC, Inferior vena cava.
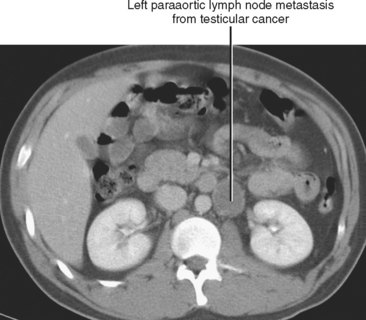
Lymphatic spread of tumor of the gastrointestinal tract is from primary nodes near the organ of origin to intermediate nodes within the ligaments and mesenteries, and eventually to the principle (preaortic) nodes. Tumors of the kidneys, adrenal glands, gonads, uterus, prostate, bladder, abdominal wall, and lower extremities commonly spread to the paraaortic lymph nodes. Tumors arising from the pelvic organs spread to the iliac lymph nodes before reaching the paraaortic nodes, whereas testicular and ovarian neoplasms spread via their draining lymphatics traveling with the gonadal vessels to the paraaortic nodes at the level of the kidneys (Fig. 10-12). As discussed later, ovarian lymphatics also drain to the iliac chains. The lymphatic drainage of the preaortic and paraaortic lymph nodes flows to the cisterna chyli (which is located anterior to the second lumbar vertebra and to the right of the aorta) and then cephalad within the thoracic duct to the supraclavicular region. Further information about the lymphatic spread of tumors is provided in this chapter in the sections that deal with individual tumor types.
BENIGN VERSUS MALIGNANT LYMPH NODES
The presence or absence of lymph node metastases is one of the most important prognostic indicators for many malignant tumors. In the case of many tumors, such as colon, rectum, gallbladder, prostate, bladder, ovarian, and endometrial cancers, the stage of nodal disease parallels the stage of the primary tumor. Unfortunately, a wide range of sensitivities and specificities have been reported for CT and MRI for detecting the presence of lymph-node metastases. Suboptimal performance of cross-sectional imaging for lymph-node staging is due to both the occurrence of micrometastases that do not alter lymph-node size or morphology and the large number of benign processes that may result in lymph-node enlargement in the absence of metastatic disease. This picture is further complicated by a lack of consensus regarding what constitutes lymph-node enlargement and controversy regarding which nodal dimension should be measured (i.e., short axis, long axis, or both). Table 10-1 gives some short-axis measurements that have been suggested to distinguish normal from abnormal lymph nodes. Table 10-2 provides an easy-to-remember rough guide to lymph-node size that can be used on screening CT or MRI scans. Note that the actual measurements used in individual practices may vary by several millimeters, and lymph-node size criteria are inversely correlated with sensitivity—that is, the larger the cutoff used to define an abnormal lymph node, the lower the sensitivity for detection of lymph-node metastases (although specificity increases). Clinical circumstances also play an important role in the assessment of lymph nodes. In recent years, there has been a trend toward viewing 8-mm (short-axis) lymph nodes in the internal iliac and obturator groups as metastatic in the setting of malignant pelvic neoplasm.
Table 10-1 Abnormal Short-Axis Lymph-Node Measurements
| Site | Nodal Size (mm) |
|---|---|
| Retrocrural | 6 |
| Paracardiac | 12 |
| Gastrohepatic | 8 |
| Porta hepatis | 7 |
| Portacaval | 10 |
| Upper paraortic | 9 |
| Lower paraortic | 11 |
| Mesenteric | 5 |
| Pelvic | 10 |
| Inguinal | 15 |
From Koh DM, Hughes M, Husband JE: Cross-sectional imaging of nodal me-tastases in the abdomen and pelvis, Abdom Imaging 31:632-643, 2006, by permission.
Table 10-2 Easy-to-Remember Approximation for Normal Lymph-Node Short Axis
| <5 mm | <10 mm | <15 mm |
|---|---|---|
| Retrocrural | All others | Inguinal |
| Mesorectal | Portocaval |
Table 10-3 presents some additional features that may help determine the likelihood that a lymph node is either benign or malignant. No individual feature is 100% specific for lymph-node metastasis, and for every feature of a malignant node there exists a benign mimic.
Table 10-3 Features of Benign and Malignant Lymph Nodes
| Characteristics | Benign | Malignant |
|---|---|---|
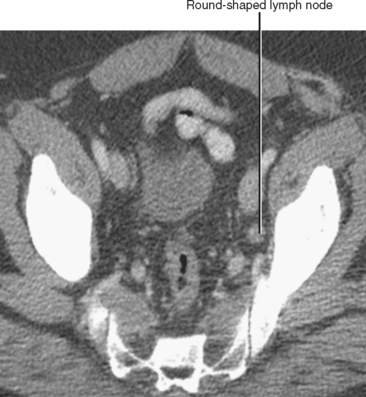
| Shape | Oval | Round (Fig. 10-13) |
| Borders | Smooth | Irregular |
| Number | Few, scattered | Numerous, clustered |
| Fatty hilum | Present | Absent |
| Consistency | Homogeneous/solid | Heterogeneous/necrotic (Fig. 10-14) |
| Confluence | Clearly separate | Confluent/matted |
| Temporal stability | Stable or shrink | Grow |
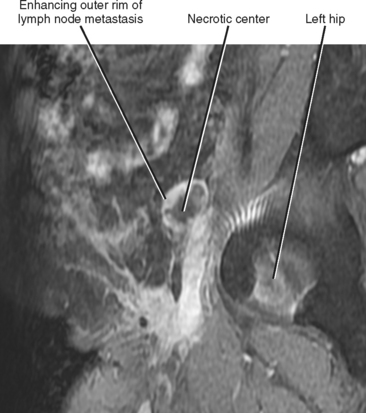
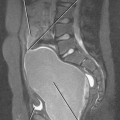
Figure 10-14 Sagittal gadolinium-enhanced magnetic resonance imaging of lymph-node metastasis in a patient with vulvar cancer.
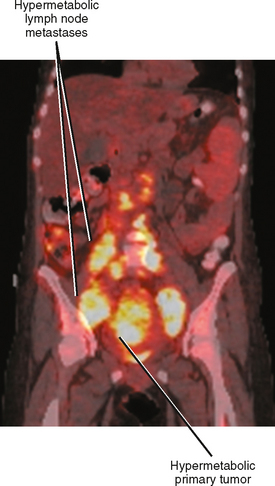
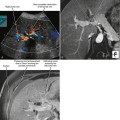
Positron emission tomographic (PET) imaging, particularly PET/CT may offer some improvement in specificity over CT and MRI alone, although sensitivity for microscopic lymph-node metastases is still lacking. Fluoro-2-deoxy-D-glucose (FDG) PET imaging detects malignant lymph nodes by demonstrating increased glucose metabolism (Fig. 10-15). Therefore, PET criteria are not based on nodal morphology or size. However, not all types of tumor are FDG avid. Some evidence suggests that increased FDG uptake in certain primary tumors such as gastric cancer and colorectal cancer may indicate an increased risk for lymph-node metastases, although PET may have difficulty in distinguishing between perigastric lymph nodes and a primary gastric tumor because of its poor spatial resolution.
Hematogenous Spread
Hematogenous spread of neoplasms may occur via the arteries or veins. Embolic spread occurs when malignant cells separate from the primary tumor and migrate to distant, noncontiguous sites via the blood vessels. Within the abdomen, the liver and adrenal glands are a particularly susceptible destination for hematogenous embolic metastases (Fig. 10-16; Boxes 10-1 and 10-2). Additional organs prone to hematogenous metastatic disease include the lungs and bones (Figs. 10-17 and 10-18). Visualized portions of these organs should be carefully scrutinized in every patient with known malignant tumor. Hematogenous spread to the gastrointestinal tract is uncommon but does occur. Malignant melanoma and carcinoma of the breast or lung are the most common sources of bowel metastases, with the most frequent sites of involvement being the stomach and small intestine (Fig. 10-19). Metastases to the spleen, kidneys, and pancreas are relatively uncommon but will eventually be encountered in a typical practice. Some tumors, such as malignant melanoma, have a reputation for metastasizing virtually anywhere via the hematogenous pathway. Unfortunately, a thorough scouring of the literature will ultimately reveal that virtually every malignant tumor has metastasized to virtually every organ at some time or another.
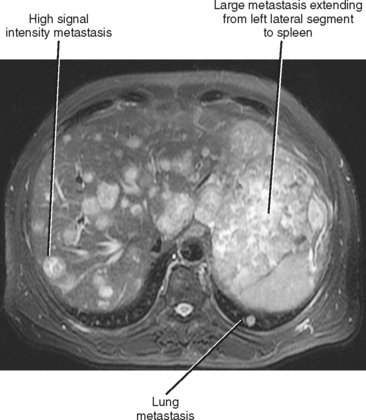
Figure 10-16 Axial T2-weighted magnetic resonance image of a patient with metastatic sarcoma involving the liver and lungs.
BOX 10-1 Common Sources of Hematogenous Liver Metastases
| Lung | Breast |
| Gastrointestinal tract | Melanoma |
| Pancreas | Gastrointestinal stromal |
| tumor |
BOX 10-2 Common Sources of Hematogenous Adrenal Gland Metastases
| Lung | Thyroid |
| Breast | Kidney |
| Gastrointestinal tract | Melanoma |
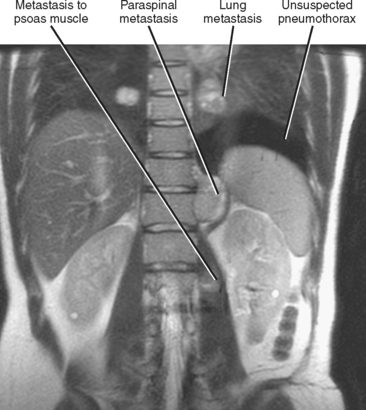
Figure 10-17 Coronal, T2-weighted, single-shot, fast spin-echo, magnetic resonance image of a patient with metastatic sarcoma.
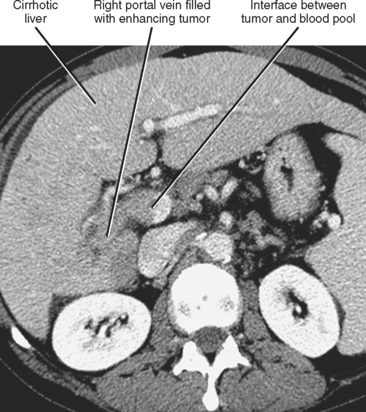
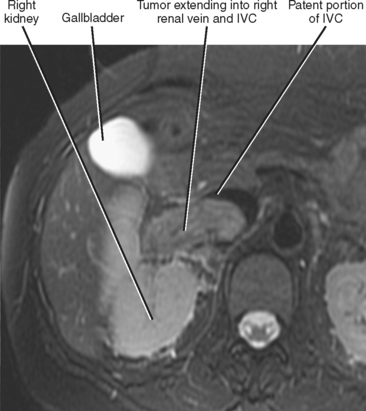
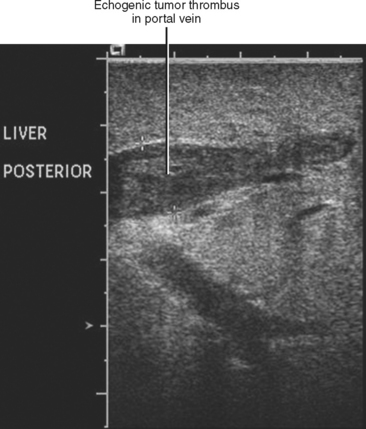
Gross contiguous extension of tumor via the lumen of a blood vessel may also occur (tumor thrombus). This latter mechanism of tumor spread is particularly common with, but not exclusive to, hepatocellular carcinoma (HCC) and renal cell carcinoma (Figs. 10-20 and 10-21).
Peritoneal Spread
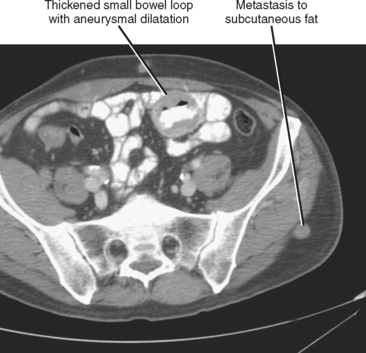
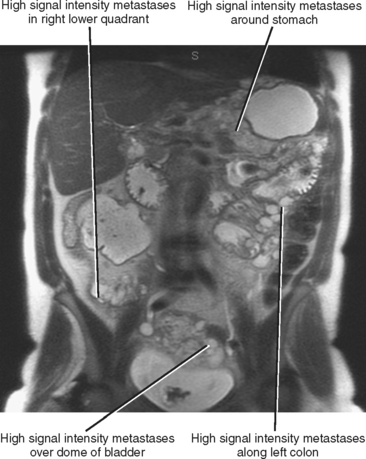
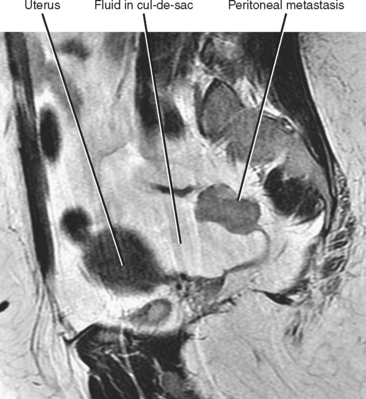
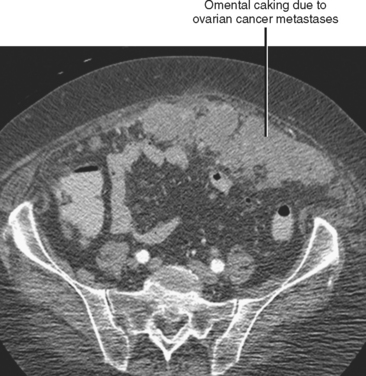
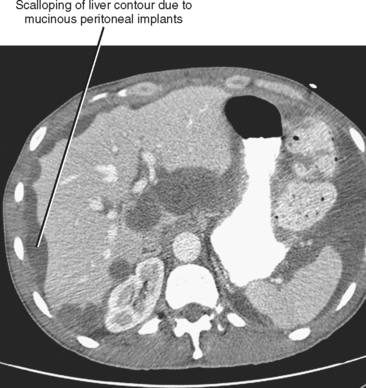
Often referred to as peritoneal seeding, peritoneal spread of tumor results from neoplastic cells breaking through the peritoneal lining and entering the coelomic cavity (Fig. 10-22). Much of our understanding of the intraperitoneal spread of malignant tumor can be attributed to the groundbreaking work of Dr. Morton Meyers, who demonstrated that the distribution of these malignant cells within the peritoneal cavity is dependent on the natural flow of ascites. The flow of ascites is dependent on gravity, changes in intraabdominal pressure, and peritoneal reflections. Peritoneal reflections impose limitations on the distribution of ascitic fluid and create recesses in which static fluid accumulates. The posterior parietal attachments are particularly important anatomic barriers to the intraperitoneal spread of tumor. The pathways of fluid flowing in the peritoneal cavity are from the right supramesocolic compartment and right and left inframesocolic compartments to the pelvis. Flow from the pelvis is to the right and left paracolic gutters. Common sites of peritoneal seeding are the rectovesical or rectouterine pouch (Fig. 10-23), the right lower quadrant along the small-bowel mesentery and its insertion near the cecum, along the sigmoid mesocolon, in the posterior subhepatic recess (Morison’s pouch), and along the right paracolic gutter. Peritoneal metastases are also relatively common in the perihepatic and subphrenic spaces (right greater than left because the phrenicocolic ligament impedes the cephalad flow of fluid on the left), and on the greater omental surface (Fig. 10-24). It is believed that the presence of lymphatics that absorb peritoneal fluid predisposes this latter structure to peritoneal metastatic implants.
Box 10-3 lists some of the more common primary sites of origin of peritoneal metastases, although metastatic peritoneal implants may be seen with a variety of other tumors with lesser frequency.
BOX 10-3 Common Primary Sources of Peritoneal Metastases
| Ovaries* | Colon |
| Appendix* | Pancreas |
| Stomach | Biliary tumors |
* Most patients with new peritoneal metastases without previously known primary malignant neoplasm will have ovarian or appendi-ceal carcinoma.
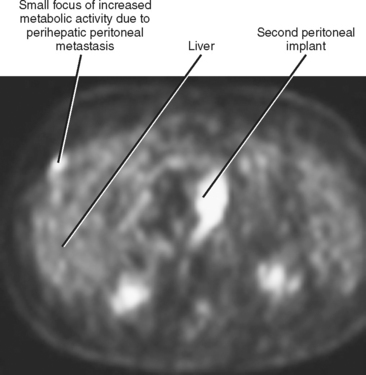
Peritoneal metastases may appear on PET as diffuse uptake throughout the abdomen and pelvis, obscuring visceral outlines, or discrete, randomly distributed foci of uptake unrelated to solid organs or nodal stations (Fig. 10-25). Ascites that occurs in the setting of one of the tumors listed in Box 10-3 is concerning for (but not diagnostic of) peritoneal metastases. Visceral peritoneal metastases are suggested on CT or MRI by scalloping of organ contours (Fig. 10-26) or bowel wall thickening or distortion. The presence of enhancing areas of thickened parietal peritoneum or nodules and plaques along the parietal peritoneal surfaces also suggests intraperitoneal spread of tumor.
Other Types of Abdominal Tumor Spread
A few final conduits of tumor spread are worthy of mention. Biliary tumors (and rarely nonbiliary tumors) may extend via the bile ducts (intraductal growth pattern). Transitional cell carcinoma may spread via the urinary collecting system, accounting for some synchronous primary urothelial tumors. Perineural spread is a relatively common type of subperitoneal extension that is often present on pathologic specimens but rarely appreciated on imaging studies. Malignant tumor has even been known to spread between the central nervous system and the peritoneal cavity via shunt tubing.
INTRODUCTION TO TUMOR STAGING
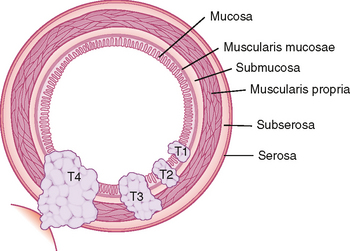
Various tumor staging systems are in use throughout the world. Accurate staging is critical to determining potential treatment options and patient prognosis, and aids in standardizing clinical research trials. In the United States, the TNM staging system of the American Joint Committee on Cancer (AJCC) is in common use among pathologists, surgeons, and oncologists. The T, N, and M included in this staging system refer to tumor, lymph node and metastasis. Figure 10-27 provides an example of T stage for cancer of the colon and rectum. This system does not take into account the degree of cellular differentiation or biological behavior of the tumor but instead relies primarily on easily determined features such as tumor size and depth of invasion. Because complete and accurate assessment of TNM stage is not possible with any imaging modality, it is most important for radiologists to be familiar with those aspects of staging with which they may be of assistance (e.g., detection of advanced stage disease that would preclude surgical resection). Therefore, we do not include a detailed description of TNM classification for every tumor. Rather, we include the aspects of staging relevant to diagnostic imaging for those tumors most likely to be encountered on a routine basis. In most cases, we do not discuss early-stage disease (e.g., T1 and T2) that can be difficult to differentiate with cross-sectional imaging. Instead, we focus on the imaging findings of more advanced disease that can significantly alter management. For more detailed staging information, we recommend one consult the latest edition of the AJCC Cancer Staging Manual. The following section attempts to provide the most upto-date information regarding staging of tumors of the abdomen and pelvis. Be aware, however, that cancer staging remains in evolution to keep pace with advances in diagnosis and treatment.
GUIDE TO SPECIFIC TUMORS
Hepatocellular Carcinoma
Common Mechanisms and Sites of Spread
HEMATOGENOUS SPREAD
Liver, lung, lymph nodes, adrenal glands, ovaries, and bones (osteolytic, see Fig. 10-18) are potential metastatic sites. Venous extension is common into the portal vein (see Figs. 10-20 and Fig. 10-28). Hepatic vein extension is less common and can extend above the diaphragm.
Key Structures to Assess
The portal and hepatic veins should be assessed for evidence of tumor invasion. HCC is frequently multifocal; therefore, it is important to look for additional tumors. When tumor resection is contemplated, it may be helpful to determine the volume of liver to remain as a percentage of the total liver volume. A residual volume of less than 25% to 30% may be insufficient to sustain the patient after resection. Invasion of the diaphragm, peritoneal metastases, or unfavorable vascular or biliary anatomy may preclude resection and should be reported when present. It is helpful to alert the surgeon to imaging findings of cirrhosis because the degree of liver failure present has a significant impact on whether a patient will tolerate surgery for HCC. Currently, the severity of liver failure is assessed according to the Child–Pugh classification, which is based on clinical and laboratory assessment.
Gallbladder Cancer
Common Mechanisms and Sites of Spread
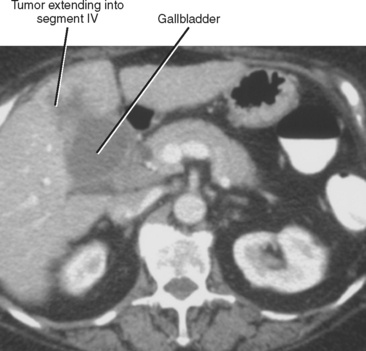
CONTIGUOUS SPREAD
Direct invasion is a common mode of spread to adjacent liver segments, most commonly segments IV and V (Fig. 10-29). Gallbladder cancer also can spread directly to the duodenum, stomach, transverse colon, and pancreas.
Perihilar Cholangiocarcinoma
Imaging Modalities
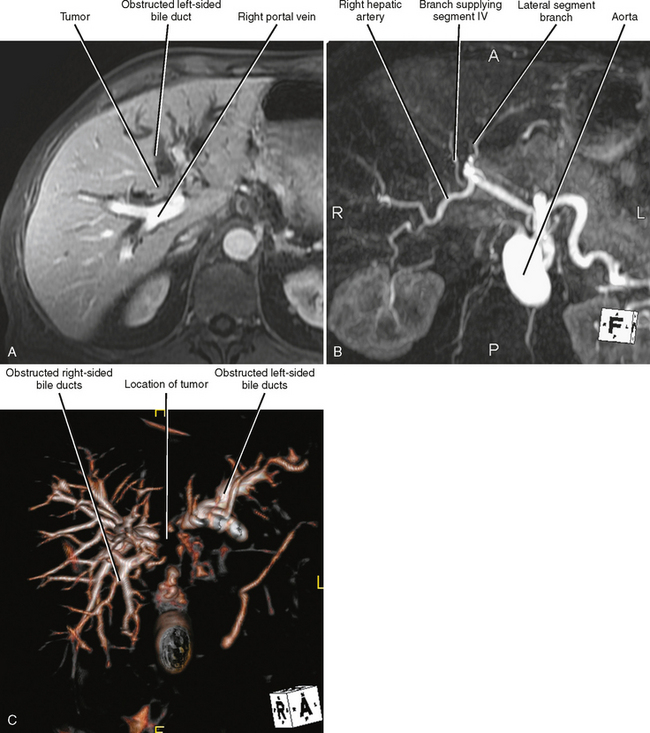
US, CT, and MRI all play a role in the evaluation of cholangiocarcinoma. MRI has the capacity to provide a combined evaluation of the hepatic vasculature, hepatic parenchyma, bile ducts, and remainder of the abdomen (Fig. 10-30). Intraoperative US may facilitate resection. US, MRCP, CT cholangiography, or percutaneous transhepatic cholangiography may be beneficial in assessing extent of duct involvement. Endoscopic retrograde cholangiopancreatography (ERCP) is commonly used for diagnosis and biliary decompression, but we recommend that cross-sectional imaging be performed before stenting because decompression of the bile ducts and stent-induced inflammation may compromise staging. The role of PET has not been established for cholangiocarcinoma.
Staging Highlights
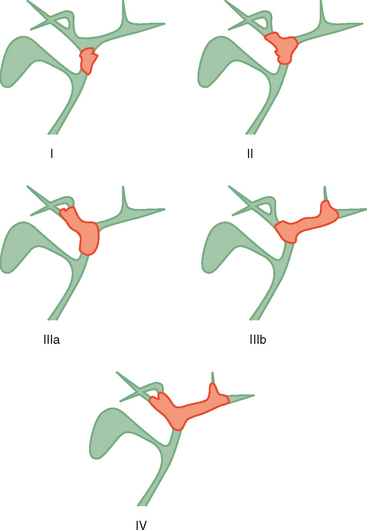
Two thirds of cholangiocarcinomas are perihilar in location. The Bismuth–Corlette classification of hilar tumors remains in common use (Fig. 10-31). Staging is also based on the TNM system. Subepithelial spread beyond gross tumor margins is common and difficult to detect with imaging. Therefore, understaging of cholangiocarcinoma is relatively common with imaging. Because surgical resection offers the best chance of cure, many surgeons will err on the side of resectability when imaging findings are inconclusive.
Common Mechanisms and Sites of Spread
CONTIGUOUS SPREAD
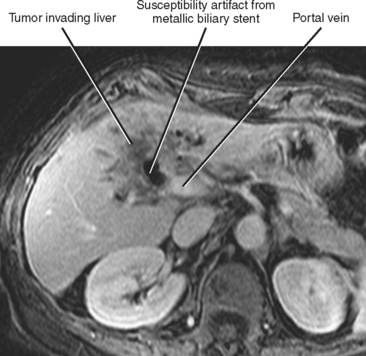
Perihilar tumors may spread by periductal infiltration or intraductal growth. Direct invasion of hepatic parenchyma is also relatively common (Fig. 10-32).
HEMATOGENOUS SPREAD
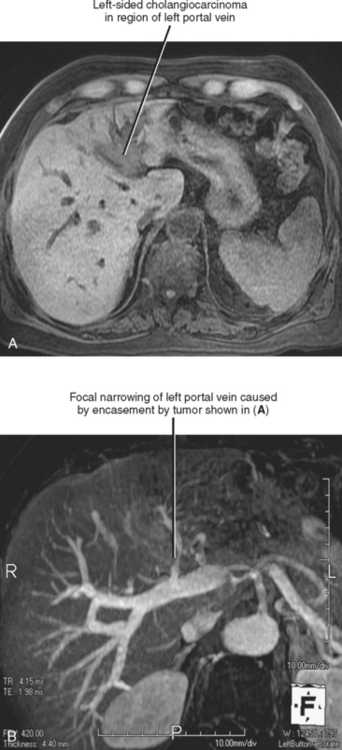
With the exception of the liver, hematogenous spread to distant sites is uncommon at time of presentation. The most frequent sites of distant recurrence after resection are the lungs and bones. Cholangiocarcinoma tends to encase the portal vein and may occasionally invade it (Fig. 10-33).