1
A Century of Brachytherapy
(From the Prostate’s Perspective)
Jesse N. Aronowitz
Brachytherapy has played a major role in the treatment of cancer, and its history could easily fill a volume; it would be inappropriate to attempt to compress it into a single chapter. I have endeavored, instead, to chronicle the story of prostate brachytherapy, which is reflective of the history of brachytherapy as a whole.
BRACHYTHERAPY: THE PREQUEL
As the discovery of X-rays and radioactivity has been exhaustively recounted (1,2), only a brief synopsis is attempted here. Wilhelm Röntgen, professor and director of the Physical Institute at the University of Würzburg, discovered in 1985 previously undescribed rays exiting a cathode-ray tube.a Within months of his discovery of the unknown (“X”) rays, they were being used for medical diagnosis and therapy.
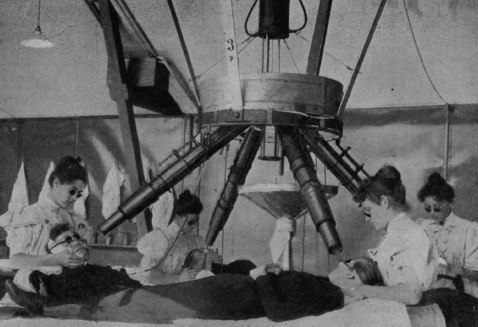
Technically, radiotherapy preceded the discovery of X-rays. Danish physician Nils Finsen demonstrated in the 1890s that lupus vulgaris (tubercular skin lesions) could be eradicated by ultraviolet (UV) light (3) (Figure 1.1).b Röntgen’s rays, a more powerful form of invisible light, were soon used in the place of Finsen’s rays. Lupus vulgaris responded, as did other dermatologic disorders; the eradication of skin cancer (rodent ulcer) by X-irradiation was reported in 1899 (4). Several radiotherapy texts were published within a decade of Röntgen’s discovery (5–7).
Figure 1.1 Finsen’s phototherapy apparatus (3). UV-rich rays from a central carbon arc lamp were directed through four sets of focusing lenses contained in brass tubes. The high-amperage lamp was rigged to treat four patients concurrently, as an economy measure. Rock crystal lenses were used, as they absorb less UV light than glass does. Water circulated among the lenses to absorb infrared rays, preventing thermal burns. Patients underwent daily treatment sessions over a period of months (a situation analogous to modern radiotherapy; public domain). UV, ultraviolet.
Source: From Ref. (3). Bie V. Remarks on Finsen’s phototherapy. Br Med J. 1899;2(2022):825−830.
Antoine Henri Becquerel discovered that uranium spontaneously emitted rays similar to Röntgen rays (1896). In 1898, graduate student Marie Sklodowska Curie identified polonium and radium, two radioactive elements present in minute quantities in uranium ore. Radium seemed to emit an inexhaustible supply of energy, and engendered an entirely new frontier in physics (8). Although radium rays were soon found to have biological properties similar to those of X-rays (the first reported radium cancer cure was in 1903 [9]), its scarcity rendered it almost unobtainable by clinicians. While X-ray tubes were cheap, radium was the most precious material on Earth (10).c The widespread practice of brachyradiumd could not become established until the element became more plentiful.
THE RADIUM INDUSTRY
The richest known deposit of uranium ore during the first two decades of the 20th century was in St. Joachimsthal (the St. Joachim Valley) in Bohemia (now Jachymov, in the Czech Republic). St. Joachimsthal’s mineral riches had been exploited for centuries; so much silver was taken from the valley that the Austro-Hungarian Empire established a mint there.e Its miners had long been known to succumb to Bergkrankheit (mountain sickness); it would be centuries before the illness was identified as lung cancer, caused by the inhalation of radioactive dust and gas (11).
Although pitchblende ore is almost 50% uranium, radium makes up only about one part per million. Tons of uranium ore were processed (through a painstaking process of chemical reactions and fractional crystallizations) to obtain a single gram of radium. Several European firms (Chininfabrik Braunschweig in Germany; Armet De Lisle and the Société Centrales des Produits Chimiques in France) produced radium commercially. The cost of radium rose after the Austro-Hungarian government restricted the export of pitchblende, and the situation worsened with the outbreak of the First World War. German physicians sought a substitute in mesothorium (a mixture of 228Ra and 228Ac), the decay product of thorium.f The French discovered radium in the American West; southwestern Colorado and southeastern Utah have deposits of carnotite, a uranium/vanadium ore (13). Although comparatively radium poor (it is only about 2% uranium), carnotite was the best available source. The ore was brought by rail to Buffalo, NY for initial extraction, and the partially processed material was shipped to France for refining (14).
Large-scale American production of radium began with the Standard Chemical Company of Pittsburgh, in 1913. Brothers Joseph and James Flannery (who were originally undertakers) had become wealthy producing vanadium for strengthening steel.g The Flannerys’ interest turned to radium after they were unable to obtain the substance in the United States for treatment of a cancer-stricken relative. When they learned that the carnotite that they had been mining in Colorado contained traces of radium, they shipped the ore to a reduction mill south of Pittsburgh for radium extraction.h Within a few years, Standard Chemical produced more than half the world’s radium (Figure 1.2). Rich uranium deposits were discovered in the Katanga province of Belgium’s Congo colony in 1915, but were not mined until after the war. The Belgians, exploiting the Congo’s rich ore and native labor, were able to halve the cost of radium, eliminating American competition.i The cost was further reduced a decade later, when rich pitchblende deposits were discovered in the Canadian Northwest Territories.
The Era of Intracavitary Radium Therapy
Prostate cancer was rarely diagnosed a century ago, but prostatitis, benign hyperplasia, and even tuberculosis of the prostate were treated by X-irradiation (15,16). Successful treatment of prostate cancer by X-rays was first reported in France in 1904 (17). Treatment of prostatic disease with radium was first reported in Paris, at a meeting of the Assoçiation Francaise d’Urologie in October 1909 (18). Ernst-Louis Desnos treated hypertrophy with a series of urethral and rectal applications (19). Henri Minet treated cancers of the prostate, bladder, and ureter with a silver tube containing 10 mg of radium, applied through a urethral catheter or a suprapubic cystotomy (20). Urologist Octave Pasteau and radium therapist Paul-Marie Degrais also began treating prostate cancer with intracavitary radium in 1909, but their first reports did not appear for several years (21). Pasteau’s rationale for preferring brachytherapy to prostatectomy was that “in cancer of the prostate the curative treatment by operation is in truth illusory; it is dangerous, and gives the most temporary results,” whereas these tumors are “particularly susceptible to the influence of radium” (22). They had used a silver capsule, containing 10 to 50 mg of radium sulfate, placed near the tip of a 17Fr coudé urinary catheter (Figure 1.3). Five treatment sessions, each lasting 2 to 3 hours, were delivered over 2 weeks. The series could be repeated periodically (annual maintenance treatments were prescribed for patients who had enjoyed a complete response). Desnos, Minet, and Degrais (who coauthored the first comprehensive radium therapy text in 1909 [23]) understood the need to filter caustic beta particles and soft gamma rays with a radiodense capsule, and that bremsstrahlung radiation (arising from the capsule) should be filtered by less dense material (rubber tubing).
Figure 1.2 One of the highlights of Marie Curie’s first trip to the United States (1921) was a visit to the Standard Chemical Company’s Canonsburg facility. Curie, seen here with company officials, appears weary, perhaps due to the radium-induced aplastic anemia to which she would eventually succumb.
Source: Photograph courtesy of the National Institute of Standards and Technology.

Figure 1.3 Pasteau and Degrais’s radium-bearing urethral catheter. The catheter was slowly advanced until urine began to drip out, at which point it was withdrawn until the dripping stopped. In this way, the radium was properly positioned in the prostatic urethra.
Source: From Ref. (21). Public domain, from Pasteau O, Degrais. De l’emploi du radium dans le traitement des cancers de la prostate (The use of radium in the treatment of prostate cancer). J Urologie Med Chirur. 1913;4:341–366.
Prostate brachytherapy was performed in Vienna in 1909 (24). Rudolf Paschkis and Wilhelm Tittinger reported the case of a 32-year-old man treated with radium at the Rothschild Hospital. The patient had been admitted for urinary retention, and digital examination suggested locally advanced, unresectable cancer of the prostate. A catheter could not be passed, so cystotomy was performed, exposing a nodular, ulcerating tumor infiltrating the bladder neck. The tumor was treated with a capsule containing 4.7 mg of radium bromide applied through the bladder fistula. Treatments lasted 20 minutes and were repeated at 2 week intervals. After 10 months of treatment, the tumor had vanished and the patient was voiding through his urethra. The case was the first to have pathologically confirmed malignancy prior to treatment, and complete clinical response following it (25).
Although Hugh Hampton Young introduced his radical prostatectomy procedure for cancer in 1904 (26), he rarely performed it, as it was uncommon for patients to be diagnosed with organ-confined disease (27). Young had attended the International Congress of Medicine in London in 1913, where he heard Pasteau and Degrais present their experience with radium therapy. He acquired 102 mg of radium and developed his own system of delivering treatment through the rectum, urethra, and bladder, as well as by applying external radium placques (essentially “crossfiring” the tumor). A single application site was treated in a daily “seance” (treatment session) lasting 1 to 2 hours. Treatment sites were alternated and carefully mapped (Figure 1.4), so that no mucosal segment was irradiated twice; in this way, urethritis, cystitis, and proctitis were avoided (28). A typical course of treatment delivered 3,000 to 4,000 mg h of radium therapy. Results were gratifying; Young reported “amazing resorption of extensive carcinomatous involvement of prostate and seminal vesicles” resulting in the “disappearance of pain and obstruction . . . which is indeed remarkable” (29). He treated 500 patients with radium therapy between 1915 and 1927 (30), and his textbook of urology devoted many more pages to radium therapy than to radical prostatectomy (28).
The Era of Interstitial Radon
James Douglas, a Canadian-American mining engineer and executive, became interested in radium after losing a daughter to breast cancer. He was appalled that she had to travel to Europe to be treated with radium that had been mined in the United States. He joined with surgeon Howard Kelly (America’s leading gynecologist, one of Johns Hopkins Medical School’s “Big Four”) in lobbying Congress to nationalize American radium-bearing lands. When Congress declined to do so, Kelly and Douglas entered into a collaborative effort with the United States Bureau of Mines. They established the National Radium Institute in 1913, with Kelly and Douglas providing the capital and the Bureau supplying the mining and processing expertise. The institute leased 16 carnotite claims in Colorado’s Paradox Valley for 3 years. The ore was transported, by burro and rail, to their processing plant in Denver. Operations ceased in 1917, after 8.5 g of radium was refined. One-half gram was donated to government hospitals, and the remaining radium was divided between Kelly and Douglas. Douglas donated his 4 g to New York’s Memorial Hospital, with the stipulation that the hospital become dedicated to the treatment of cancer (31).j

Figure 1.4 Record of the dates and locations of rectal applications. A similar record was kept of urethral and bladder neck applications.
Source: From Ref. (28). Young HH. Treatment of carcinoma of the prostate. In: Young HH, Davis DM, eds. Young’s Practice of Urology: Based on a Study of 12,500 Cases. Vol 1. 1st ed. Philadelphia, PA: WB Saunders; 1926:644–671. Used with permission.
Radium’s specific activity (ratio of activity to mass) is low, due to its long half-life (1,600 years). In practical terms, it takes at least a week to deliver a curative dose with radium needles. This would be particularly awkward for the treatment of prostate cancer, as the sources would be left in an open suprapubic or perineal wound for an extended period (32). The solution to this problem lies in radon, radium’s first daughter product (Table 1.1). As most of the therapeutic gamma rays exiting a radium tube were actually emitted by daughter product “radium C” (214Bi), radium (226Ra) itself was unnecessary for “radium therapy.” Treatment with radium C would be challenging, due to its 20 minute half-life, but radon (known as radium emanation until 1923) could serve as a reservoir for radium C. Radon has a very high specific activity, owing to its short (3.8 day) half-life; despite being a gas, 1 Ci of radon has a volume of less than 1 mm3. Because of its high specific activity, an “emanation” needle could be much thinner than a radium needle. Consequently, radium salts were kept in an aqueous solution, and the emitted radon gas was harvested for therapeutic applications. Unfortunately, the collected gas was mostly composed of water vapor, hydrogen and oxygen (from electrolysis of the water), helium (from alpha particles), and chlorine (from the hydrochloric acid used to keep the radium ions in solution). Harvard biophysicist William Duane had spent 7 years as a research associate of the Curies, much of that time focusing on the purification of radon. On his return to the United States, he built a radium emanation plant at Boston’s Collis P. Huntington Hospital, which he replicated at Memorial Hospital (33,34). Memorial’s entire 4 g of radium was kept in solution (Figure 1.5), and the purified radon was encapsulated in short lengths of glass capillary tubes, 0.3 mm in diameter (Figure 1.6), which were inserted into hypodermic needles. The radon-bearing needles were used for temporary implantation (the needles’ steel filtered most beta particles and soft gamma rays).
Table 1.1 The radium-226 decay cascade

Figure 1.5 Memorial’s emanation plant. All of Memorial’s radium was kept in solution in the safe (bottom right). The emitted radon was captured and purified.
Source: From Ref. (33). Failla G. The physics of radium. In: Clark JG, Norris CC, eds. Radium in Gynecology. Philadelphia, PA: JB Lippincott Co; 1927:63. Used with permission.

Figure 1.6 Capillary glass radon tubes, inserted into “serum” needles for temporary implantation.
Figure 1.7 A gold-encased radon seed. Note the resemblance of the dimensions to modern seeds.
Beginning in 1915, Memorial’s urologist, Benjamin Barringer, used these needles for outpatient treatment of prostate cancer (35). With the patient in the lithotomy position, Barringer anesthetized the perineum prior to implanting a 15 cm needle, under the guidance of a finger in the rectum, into a lateral prostate lobe. The needle, bearing 50 to 100 mCi of radon in its distal 3 cm, was left in place for 4 to 6 hours before being retracted and inserted into the other lateral lobe. The seminal vesicles were often treated through a transrectal puncture. Treatments were repeated as necessary, at intervals of several months (36). Barringer reported highly favorable tumor responses.
With abundant quantities of short half-life radon, it became appealing to perform permanent implants. At first, “bare” glass tubes were implanted into tumors, but this practice resulted in painful sloughing of necrotic tissue. Memorial’s physicist, Gioacchino Failla, recognized the offender to be unfiltered caustic beta particles. He remedied the problem by encasing the radon in a 0.3 mm thick envelope of gold (Figure 1.7) that filtered out 99% of beta particles while allowing more than 80% of therapeutic gamma rays to pass (37). Barringer implanted up to 20 seeds, each containing 1.5 to 2.0 mCi of radon, into the prostate, typically delivering 4,000 mCi h of treatment (38). Barringer’s techniques were adopted at other institutions (39,40), and a “gold” radon seed industry was established that persisted in the United States for decades (Figure 1.8) (41).k
Introduction of Man-Made Radionuclides
The large majority of prostate cancer patients undergoing radium or radon brachytherapy developed recurrence (38). This is not surprising, as most men diagnosed in that era had advanced disease that could not be cured by any means. The use of prostate brachytherapy waned after Charles Huggins (1901–1997) discovered that prostate cancer responds to androgen deprivation (1941) (42), but interest revived on recognition that castration was only temporarily effective.
Congress passed the Atomic Energy Act after World War II, establishing the Atomic Energy Commission. The Oak Ridge Laboratories were transferred to civilian control and directed to provide radioisotopes for peaceful purposes, including medical applications. One of the first radionuclides made available was radiogold (Au-198); its short half-life (2.7 days) is comparable to that of radon, but is safer to handle because it does not generate megavoltage photons and has no radioactive daughter products. Microparticles of radiogold were suspended in pectin or gelatin, forming a colloid for instillation into pleural or peritoneal cavities (to suppress malignant effusions and ascites) (43), or injected into lymphomatous masses and solid tumors (44). The first radiogold prostate implant, at the University of Iowa in 1951, was unplanned (45). The prostate of an 80-year-old man with hormone-refractory Stage C disease was surgically exposed for radon seed implantation, but the seeds were not available. Colloidal gold was at hand, and was infiltrated into the prostate. The treatment was without apparent toxicity, the bulky tumor resolved, and follow-up biopsy was negative (46). The urologist, Rubin Flocks, began infiltrating colloidal gold into the prostate and seminal vesicles of men with Stage C disease, through suprapubic and perineal approaches. An enthusiastic report on 20 cases was published in the Journal of Urology the following year (47).

Figure 1.8 The American Association of Genitourinary Surgeons has honored Barringer by awarding the Barringer Medal for outstanding achievement in urology since 1955. Note the symbols of radioactivity, the trocar, and “seeds.” Used with permission of the American Association of Genitourinary Surgeons.
There were compelling reasons to consider colloidal gold as a suitable radionuclide for prostate brachytherapy. It is a beta emitter that deposits 90% of its energy within millimeters (it was assumed that the fascia investing the prostate and seminal vesicles would limit the colloid’s migration). There was evidence that gold microparticles would be phagocytosed by macrophages, which, on circulating to draining lymph nodes, would irradiate D1 metastases (48). In reality, treatment did not work as expected. Dense tumor nodules resisted infiltration; the colloid had to be injected under pressure, resulting in spattering that contaminated drapes, scrubs, and shoes (Figure 1.9). Radiation exposure to personnel was so high that surgical teams were rotated to avoid accumulation of prohibitive doses (49). Much of the injected material leaked out of the prostate, pooling in the pararectal gutters, causing severe rectal injury (50). Some of the gold microparticles entered the circulation, and autoradiographs demonstrated hepatic accumulation. Although radiogold did percolate through regional lymphatics, it did not penetrate tumor-congested nodes (50). Flocks devised several maneuvers to overcome these difficulties: Grossly abnormal lymph nodes were resected; hyaluronidase and epinephrine were mixed into the colloid to improve distribution and reduce vascular uptake; and small volumes of highly concentrated suspension were used to reduce leakage from the gland (49). It became apparent that the procedure was only effective for the smallest tumors (50), and 80% of posttreatment biopsies were positive (51). Flocks eventually resorted to perineal prostatectomy, using radiogold as adjuvant therapy (infiltrating the colloid into periprostatic fascia and vascular pedicles) (46). He defended the procedure, claiming better local control (95%) for Stage C disease, compared to published prostatectomy series (70%–80%) (52). Toxicity, however, was significant: delayed healing in 80% and “persistent urethro-cutaneous fistula” in 2% (53). Use of colloidal gold continued at the University of Iowa Hospitals until its manufacture ceased in 1977; thereupon, radiogold grains were substituted.

Figure 1.9 Device used at the University of Iowa to inject colloidal gold under pressure. It was heavily shielded, to reduce the operator’s exposure.
Source: From Ref. (49). Flocks RH, Culp DA. Radiation Therapy of Early Prostate Cancer. Springfield, IL: Charles C. Thomas; 1960. Public domain.
Urologist C. Eugene Carlton (1930−) initiated a prostate brachytherapy program at Baylor Hospital in 1965. He chose to implant Au-198 grains (rather than colloid) because of ease of handling and more accurate placement. The procedure began with lymph node dissection, followed by incision of the endopelvic fascia and mobilization of the prostate, allowing implantation under direct visualization (54). Initially, a single gold grain was implanted in the tumor nodule; eventually, the procedure entailed implantation of 6 to 10 grains, distributed within the gland (55) (Figure 1.10). Cobalt (later, linac) teletherapy began 2 to 3 weeks later; the radio-opaque grains served as fiducial markers, identifying the prostate. Although the procedure was designed for Stage C disease, early results were so promising (58% negative biopsies) that it was also used to treat patients with organ-confined disease (54). Toxicity consisted mostly of thrombophlebitis and temporary extremity or genital edema (secondary to the node dissection). The incidence of proctitis was 16%, but less than 1% of patients required colostomy (56). Impotence was reported to develop in only 5% of men who were potent prior to treatment. The procedure became so popular that many private urologists at affiliated hospitals participated. But the Baylor program had serious flaws. The radiogold grains were delivered to Baylor once weekly, and their activity at time of implantation varied widely (between 2 and 9 mCi, depending on the day of implantation). Dosimetry was crude; dose was estimated by assuming that the entire implant activity was deposited at the geometrical center of the prostate, and the delivered dose was defined as the isodose that subtended a diameter equivalent to that of the gland (56). It is difficult to encompass the gland with so few sources, even if they were well placed. Years later, formal dosimetric evaluation demonstrated that these implants typically delivered less than a third of the prescribed dose (57). It is not surprising that, with longer follow-up, treatment outcomes were disappointing (58,59).

Figure 1.10 A Baylor implant. It is difficult to achieve adequate coverage with only six sources, even with a high energy radionuclide.
Source: From Ref. (55). Hudgins PT. Irradiation of prostatic cancer combined with abdominal exploration. In: Fletcher GH, ed. Textbook of Radiotherapy, 2nd ed. Philadelphia, PA: Lea & Febiger; 1975:768–772. Used with permission.
Ulrich K. Henschke (1914–1980) came to New York to head Memorial’s brachytherapy service in 1955. He had spent the previous 3 years at Ohio State University, where he collaborated with William Myers in the introduction of Au-198 and Ir-192 into clinical practice (60,61). In New York, however, most of his permanent implants used radon, because the daily seed requirement was unpredictable (he was called to the operating room whenever a surgically exposed tumor was found to be unresectable) (62) and large quantities of radon seeds were produced by Memorial’s radon plant. In 1963, health physicist Donald Lawrence sought funding from Memorial for production of an I-125-impregnated suture (63). Henschke provided encouragement and modest financial support, but advised that the radionuclide be encapsulated in a seed.l Within months, Lawrence sent him iodine seeds for animal studies, and Henschke began performing human I-125 implants for lung cancer in 1965 (64). Henschke’s protégé, Basil Hilaris (1928−), assumed leadership of the brachytherapy service upon Henschke’s departure in 1967. Brachytherapy had been used at Memorial as salvage therapy for locally recurrent prostate cancer (following failed radiation or hormonal therapy) since 1956 (65), and Hilaris proposed I-125 prostate brachytherapy as primary treatment to Memorial’s chief of urology, Willet Whitmore, Jr. (1917–1995). Whitmore was receptive; he had attempted aggressive surgical resections early in his career, but by 1963 (66), acknowledged the futility of radical prostatectomy to control locally advanced disease. He concluded that intervention for prostate cancer should consider quality of life, and “need not necessarily involve an effort at cancer cure” (67).
Memorial’s I-125 implant procedure began with the patient in a modified lithotomy position (68). A Foley catheter was inserted and an O’Connor drape (with an appendage allowing insertion of a finger into the rectum) was placed. A midline or paramedian incision extended from the umbilicus to the pubis. External, hypogastric, and obturator nodes were dissected. Fat was removed from the anterior surface of the prostate, but the puboprostatic ligaments were left intact. The endopelvic fascia was incised, mobilizing the lateral margins of the gland, but the prostate was not dissected from the rectum (69). The radiation therapist then inserted empty 15 cm long 16-gauge steel needles into the gland, spaced approximately 1 cm apart. The needles were slowly advanced until sensed by a finger in the rectum (Figure 1.11). Gland dimensions, measured intraoperatively, were used to calculate prostate volume, which determined total implant activity. The number of seeds needed for the implant was derived by dividing the calculated total implant activity by the activity of the available seeds (the ideal seed strength was eventually determined to be 0.5 mCi). Memorial physicist Lowell Anderson developed nomograms to rapidly calculate seed requirements and spacing (70). An applicator was developed to implant the seeds (71) (Figure 1.12). Bleeding could be heavy (median blood loss was 1 L), and almost half the patients required transfusion (72). The Foley catheter was removed 1 to 3 days postoperatively, and the patient was discharged a week later. Postoperative irradiation was delivered to patients found to have lymphatic metastases or bulky tumors (73). Operative mortality was rare (0.5%). The most distressing complications (venous thrombosis, pulmonary embolism, lymphocele, lymphedema) were attributed to lymph node dissection. Impotence or incontinence occurred in fewer than 10% of cases (72).
A computer program was used to calculate dose distribution from postimplant radiographs (see the “Computer Dosimetry” section; Figure 1.13). Without accurate delineation of the target volume, however, the adequacy of an implant was difficult to determine. The dose covered by a volume equivalent to that of the prostate (calculated from intraoperative measurements) was deemed the “matched peripheral dose” (mPD; Figure 1.14) (74). This metric was misleading, as there was no indication that the target and the treated volumes coincided (75) (published radiographs suggest that they often did not [68]), and intraoperative measurement was later found to underestimate prostate volume (76). Disease control was monitored by digital examination, acid and alkaline phosphatase levels, and bone scans. Local control (as determined by palpation) was 80% at 5 years if the mPD exceeded 10,000 rads (100 Gy) (74). Of the 40% of patients found to have nodal metastases, fewer than half survived 5 years, and were found not to have benefited from nodal dissection or irradiation; thereafter, nodal dissection was eliminated from the procedure (73).

Figure 1.11 Retropubic implantation of needles toward a finger in the rectum.
Source: From Ref. (67). Whitmore WF Jr. Proceedings: the natural history of prostatic cancer. Cancer. 1973;32(5):1104–1112. © 1975 Memorial Sloan-Kettering Cancer Center. Used with permission.
Figure 1.12 Seed implantation using an early applicator designed by Felix Mick (when he was employed by Memorial Hospital).
Source: From Ref. (71). Hilaris BS. A Manual for Brachytherapy. 2nd ed. New York, NY: Memorial Hospital; 1970:65. © 1970 Memorial Sloan-Kettering Cancer Center. Used with permission.

Figure 1.13 Computer-generated dose distribution from an open retropubic implant.
Source: From Ref. (68). Hilaris BS, Whitmore WF, Batata M, et al. Cancer of the prostate. In: Hilaris BS, ed. Handbook of Interstitial Brachytherapy. Acton, MA: Publishing Science Group; 1975:219–234. © 1975 Memorial Sloan-Kettering Cancer Center. Used with permission.
Figure 1.14 Determination of matched peripheral dose (mPD) from a computer-generated dose–volume histogram. The mPD was the dose delivered to a volume of tissue equivalent to the intraoperatively calculated prostate volume.
Source: From Ref. (68). Hilaris BS, Whitmore WF, Batata M, et al. Cancer of the prostate. In: Hilaris BS, ed. Handbook of Interstitial Brachytherapy. Acton, MA: Publishing Science Group; 1975:219–234. © 1975 Memorial Sloan-Kettering Cancer Center. Used with permission.
More than a thousand patients were implanted with iodine seeds at Memorial Hospital between 1970 and 1986. It was appreciated that quality implants controlled very early disease, but few patients had presented with early disease, and few implants delivered the prescription dose. Disease-free survival curves never plateaued (77), and reports of disappointing long-term control rates (78,79) led to abandonment of the procedure.
RETURN OF THE TRANSPERINEAL APPROACH AND INTRODUCTION OF IMAGE GUIDANCE
The template, a simple device that directed the distribution of implanted sources, appeared by mid-century (80). It improved implant quality by maintaining source spacing and parallelism (Figure 1.15) (81).
Beginning in 1971, University of Miami radiation oncologist Komanduri Charyulu (1924−) performed “closed” implants on patients with disease too advanced for the standard “Memorial” technique (82). With the patient in the lithotomy position, he passed needles through a handheld template positioned against the perineum. The template could be angled to overcome pubic arch interference. Needles were advanced, under fluoroscopic guidance, up to the contrast-filled bladder. He could not, of course, visualize the prostate by fluoroscopy, but his object was to encompass the region of the prostate with a matrix of seeds, 4 cm wide, 4 cm high, and 5 cm deep (Figure 1.16). Charyulu’s plan utilized three strengths of radon seeds (0.15, 1.0, and 0.8 mCi) in a Paterson−Parker distribution, to achieve a relatively homogeneous dose distribution. Charyulu’s transperineal patients enjoyed superior local control, without surgical complications, compared to patients with earlier disease that he had treated with the Memorial “open” retropubic technique.
Figure 1.15 An acrylic radium needle “stabilizer,” predecessor to the template.
Source: From Ref. (81). Green A. New techniques in radium and radon therapy. J Fac Radiol. 1951;2(3):206–223.

Figure 1.16 A Charyulu implant. Finally, an implant that looks like it might work.
Source: From Ref. (82). Charyulu KKN. Transperineal interstitial implantation of prostate cancer: a new method. Int J Radiat Oncol Biol Phys. 1980;6:1261–1266.
At the University of Nebraska in 1979, Pradeep Kumar began implanting I-125 seeds transperineally (83). The seed requirement (to achieve a minimal prostate dose of 160 Gy) was estimated preoperatively from a CT scan. The patient was placed in the “semi-lithotomy position” with contrast in the bladder. A guide needle, without a flange, was passed anterior to the anus and rectum, under direction of a finger in the rectum. A template was slid over this needle, and implant needles were inserted through the template in a triangular pattern (defined by the pubic arch and rectum). Needle insertion was directed by fluoroscopy. Approximately 50 I-125 seeds (0.3–0.5 mCi each) were implanted with a Mick applicator (Mick Radio-Nuclear Instruments, Mount Vernon, NY) resulting in an average minimal peripheral dose (as calculated from postoperative orthogonal films) of 154 Gy (84). A 5-year local control was reported to be 85% (85). Kumar began implanting seeds in braided absorbable sutures (obtained from the 3M Corporation, St. Paul, MN) in 1983 (86). This approach maintained seed spacing and allowed placement of extracapsular seeds without the risk of seed migration. The procedure time was reduced to 45 minutes (87), and prostate brachytherapy was offered as an outpatient service in 1987 (85).
Memorial Sloan-Kettering brachytherapists transitioned from “open” retropubic implants to transperineal implants in the 1980s. Patients underwent a planning CT scan with an obturator in the rectum (88), and total the activity of the implant was determined by a nomogram (89). A custom acrylic template, with holes drilled according to the treatment plan, was fabricated for each case (Figure 1.17) (90). Patient positioning was recapitulated in the operating theater with the rectal obturator attached to the perineal template. Needles were inserted under fluoroscopic guidance, and seeds were implanted by a Mick applicator. Transrectal ultrasonography was incorporated into the procedure by 1990 (90).
The Incorporation of Sonography
Physicists involved in the discovery of radium also uncovered the principles underlying sonography. The piezoelectric effect (the property of certain crystals to develop an electric potential when mechanically stressed) was described in 1880 by Marie Curie’s future husband (Pierre, 1859–1906) and brother-in-law (Jacques, 1856–1941). The following year, Marie Curie’s thesis adviser (Jonas Gabriel Lippmann, 1845–1921) predicted that a change in electric potential would alter a crystal’s dimensions (91). These phenomena underlie the function of the ultrasound transducer: The generation and detection of sound waves. The first practical application of sonography was a device to detect German U-boats (sonar), patented in 1916 by Pierre Curie’s doctoral student (Paul Langevin, 1872−1946m). Sonography was later used by industry as a nondestructive method for detecting metal flaws and fatigue (replacing X-rays and gamma rays for that purpose) (92). Ultrasound was applied by physiatrists in the 1930s to therapeutically heat subsurface tissues (93). Diagnostic applications were developed in the late 1940s; initial attempts measured the transmission of ultrasound waves through tissue (hyperphonography) (94), but detection of reflected waves was investigated by 1950 (95). Sonography for detection of cancer was described in 1957 (96).

Figure 1.17 Custom template with obturator. The treatment plan specified needle angle, as well as perineal entry position and depth.
Source: From Ref. (90). Nori D, Donath D, Hilaris BS, et al. Precision transperineal brachytherapy in the treatment of early prostate cancer. Endocuriether Hypertherm Oncol. 1990;6:119–130. Used with permission of AMN Syed.
An inventive and mechanically inclined Danish surgical resident, Hans Henrik Holm (1931−), became interested in sonography during a radiology rotation. He visited physicist Carl Hellmuth Hertzn (1920–1990) in Lund, Sweden. Hertz had explored medical applications of sonography with cardiologist Inge Edler (“father of echocardiography,” 1911–2001) and neurosurgeon Lars Leksell (“father of radiosurgery,” 1907–1986). Holm was awarded a state grant to obtain an ultrasound unit, and duplicated Lund’s multidisciplinary methodology by collaborating with a cadre of young physicians, as well as with the Welding Institute (a state technology laboratory) to adapt and develop ultrasound apparatus for clinical use.o Equipment was designed to be mobile, so that bedside procedures could be performed. The group developed techniques for interventional sonography in the 1970s, including percutaneous biopsy, drainage, pericardiocentesis, amniotic fluid sampling, and percutaneous nephrostomy.
In 1974, the Welding Institute introduced a probe with transducers for transurethral and transrectal imaging (97,98). A “fixing sledge” (stepper unit) that retracted the probe at 5 mm intervals facilitated planimetric volume determinations (98). A metal template mounted on the probe shaft directed prostate and seminal vesicle biopsy (99). By 1980, Holm was using ultrasound guidance to implant I-125 seeds (separated by chromic suture spacers) into liver metastases and pancreas tumors (100,101). The prescription dose was 160 Gy, and most patients also underwent adjuvant teletherapy. By 1982, he was implanting I-125 seeds into cancerous prostates, under the direction of axial imaging from a rectal probe mounted on a sledge-stepper (102). Preplanning and implantation were performed with the patient in the lithotomy position. A modified Memorial Hospital nomogram determined the implant activity needed to deliver 160 Gy, based on Henschke’s system of dimension averaging (103). A 3 cm thick acrylic template was attached to the probe shaft (Figure 1.18). After immobilizing the gland with an empty needle passed through the template, needles preloaded with seeds and spacers were inserted. Needles that were to be advanced most deeply (in the central gland) were placed first. After proper needle position was confirmed by transverse sonographic imaging, the seeds were deposited by stabilizing the stylet while the needle was retracted. The ultrasound probe was then retracted 5 mm, and the next deepest set of needles was placed; in this fashion, concentric circles of needles were inserted and their seeds deposited. Postimplant dosimetry was performed on orthogonal radiographs the following day (Figure 1.19).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree